This webcast features: Dr. Burkhard Joksch, Product Manager, Bioprocess Automation at Sartorius Stedim Biotech GmbH, and Dr. Stuart Tindal, Product Manager, FlexAct® Platform at Sartorius Stedim Biotech GmbH Multi-product facilities need modular package units to realize fast change-over without reducing product quality and process performance. Smart modular package units such as the FlexAct® platform can run pre-qualified and pre-tested recipes, users can rapidly integrate Sartorius Stedim Biotech’s and other manufacturers’ single-use technology in bioprocess operations, achieving faster installations with reduced…
Friday, April 12, 2019 Daily Archives
Visible Particulate Matter in Single-Use Bags: From Measurement to Prevention
Parenteral pharmaceuticals must be “essentially free” from visible particulate matter (1). In the production of biopharmaceuticals with single-use systems (SUS), biocompatibility requires controlling interactions between drug substances/products and SUS surfaces to ensure drug product quality and patient safety with regard to extractables/leachables and particulate matter. Any particulate matter stuck to fluid-contacting surfaces of process components could wash off and contaminate process fluids. Depending on system configuration, a final drug product could be at risk for particulate matter from SUS. Risk…
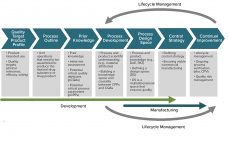
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver…
Competing for Talent: Make Your Biotech Workplace a Powerful Asset
Talent is the lifeblood of pharmaceutical innovation. But there’s more to winning top talent than simply recognizing the value of our “human resources.” It also takes putting that value into action by giving people a workplace that inspires and even delights. Today, forward-looking industry leaders are seeing competitive advantage in rethinking workplace strategy and the role it plays in attracting and retaining talent, according to a 2018 report (1). Once considered to be merely a passive background for discovery, a…
Cell therapy vendors: News from Miltenyi and Saint-Gobain
Bellicum Pharmaceuticals has inked a supply agreement with Miltenyi Biotec; Hitachi Chemical has teamed with Saint-Gobain to address manufacturing challenges – welcome to Bioprocess Insider’s cell therapy services round-up. In an SEC filing, Houston, Texas-based cellular immunotherapy firm Bellicum Pharmaceuticals revealed it has entered a agreement with Miltenyi Biotec GmbH for the supply of “certain products in connection with the development and manufacture of certain of the Company’s products.” The firm will pay Miltenyi a €2 million ($2.3 million) upfront…
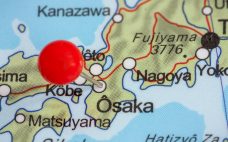
Aji Bio opens large-scale oligo production plant in Osaka
Facilitated by its 2016 acquisition of GeneDesign, CDMO Ajinomoto Bio-Pharma has opened a 21,500 square-foot plant in Japan to service the growing oligonucleotide demand. In December 2016, Japan’s Ajinomoto OmniChem acquired nucleic acid drug contract development and manufacturing organization (CDMO) GeneDesign for an undisclosed fee. Nearly two-and-a-half years on and the subsidiary has become absorbed into Ajinomoto’s Bio-Pharma Services division. “GeneDesign – now Ajinomoto Bio-Pharma Services Osaka – provides solid-phase technology for oligonucleotide synthesis capabilities from µg to 10 kg…