BioProcess International US West is the leading phase-based bioprocessing event for accelerating promising biologics toward commercial success. Whereas most bioprocessing event sessions are broken out by departmental function — e.g., analytical, upstream, and downstream — the BPI US West sessions are defined by stage of development, providing drug developers a unique opportunity to break down silos across multiple departments to discuss today’s leading solutions toward improving the speed, lowering the cost, and increasing the quality/safety of biologics in development. Each…
Tuesday, March 5, 2019 Daily Archives
Biogen buying retinal gene therapy firm Nightstar for $800m
The acquisition will give Biogen access to two potentially first-in-class later-stage gene therapy assets, NSR-REP1 and NSR-RPGR, for ophthalmologic disorders. The $800 million (€706 million) deal sees Biogen bolster its rare disease interest through the addition of Nightstar Therapeutics’ expertise in gene therapy and ophthalmology. Speaking on a conference call to discuss the acquisition, Michel Vounatsos, Biogen’s CEO, said the addition of Nightstar “fits hand-in-glove” with Biogen’s strategy and business. “This transaction will build on our progress of developing and…
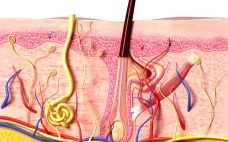
Celltrion injects $51m to get under the skin of infliximab competitors
Celltrion Pharm has pledged KRW 58.2 billion won ($51 million) to establish a production site for Remsima SC, a subcutaneous version of its Remicade biosimilar. Korean drugmaker Celltrion has achieved regulatory success for its biosimilar of J&J’s Remicade (infliximab) across several key markets and is now looking to receive approval and launch a subcutaneous version of the immunosuppressive monoclonal antibody. To prepare for launch, the firm has committed KRW 58.2 billion won ($51 million) to its site in Cheongju, South…