Following success with several biosimilars, bringing a novel biologic into the clinic is the “next logical step” in becoming a fully integrated biopharma firm, says Samsung Bioepis. Almost a year to the date it announced a co-development agreement, Samsung Bioepis and Takeda Pharmaceuticals are preparing to begin a Phase I trial for its lead candidate, SB26, also known as TAK-671. The news signals the latest stage in the development of Samsung Bioepis – a joint venture between South Korean contract…
Monday, August 13, 2018 Daily Archives
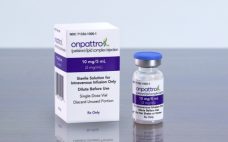
Alnylam’s success brings first RNAi therapeutic to US
The US Food and Drug Administration (FDA) has approved the first ever RNA interference (RNAi) therapy: Alnylam’s Onpattro (patisiran). Alnylam announced last week Onpattro has been approved in the US for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The product was hailed by the FDA as the first in a new class of drugs: small interfering ribonucleic acid (siRNA) treatments. “New technologies like RNA inhibitors, that alter the genetic drivers of a disease, have the…