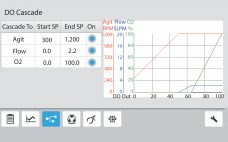
Learning to use a bioprocess controller is a complex endeavor. Even if someone has previous bioprocess experience, moving to a new software platform can entail much learning and reduce process efficiency. With the auto-culture modes of the Eppendorf BioFlo 120 bioprocess control station, a user can select with a push of a button either a predefined Escherichia coli batch fermentation protocol or a Chinese hamster ovary (CHO) batch cell culture process. Proof of Concept: E. coli Auto-Culture Fermentation The auto-culture…
Thursday, August 10, 2017 Daily Archives
Extractables and Leachables Studies: Designed and Performed to Meet All Intended Needs
There are no definitive requirements on how to perform extractables and leachables (E&L) studies. However, the US Pharmacopeial Convention recently released two chapters to provide guidance on performing E&L studies. USP chapters <1663> and <1664> discuss options and considerations in performing an extractables study and a leachables study, respectively. There are also various workgroups that have published guidance documents to assist industry in designing and performing these studies. The Product Quality Research Institute (PQRI), the Bio-Process Systems Alliance (BPSA), and…
Automation and Modularity Allow MAb Biotech to Cut Scale-Up Time
Original developers of biosolutions and products, especially those facing the debut of biosimilars in core markets, have an urgent imperative to reduce manufacturing costs through increased productivity and yields. In turn, this drives a wide range of business decisions, including capital investment, process choices and design, and equipment selection. To this end, biodevelopers are adopting more sophisticated processes (such as perfusion) to address low-titer cell lines and reduce raw material costs. They’re also seeking more sophisticated and flexible R&D and…
Looking into the Future: An Interview with Steve Bagshaw, CEO of Fujifilm Diosynth Biotechnologies
Steve Bagshaw, chief executive officer at FUJIFILM Diosynth Biotechnologies, offers some insight into the ever changing CDMO landscape and the future of medicine. Mr. Bagshaw is an industry thought leader and an advocate of the Biotechnology Industry. Q: Fujifilm Diosynth Biotechnologies has been expanding and making big announcements in the past year. Can you talk about how this growth is shaping your long-term vision as a contract development and manufacturing organization (CDMO)? We are seeing the benefits of long-term investment.…
Single-Use Diaphragm Valve
There is now a larger selection of the world’s first controllable single-use diaphragm valve: GEMĂś SUMONDO. GEMĂś, the leading manufacturer of valve designs for the pharmaceutical industry, has established the first controllable single-use diaphragm valve on the market: the GEMĂś SUMONDO. In addition to a pneumatically operated version, the product range now includes a version with a hand wheel for manual operation. And because of increased customer demand globally, the product range has been expanded in the area of associated…
Production of Biotherapeutics Using GlycoExpress® Expression Platform Technology
GlycoExpress® (GEX®) technology is a well-established human expression platform for the screening and production of biotherapeutics. By contrast with other human cell lines (e.g., HEK293 and Per.C6) GlycoExpress® expression platform consists of a toolbox of glycoengineered cell lines optimized for producing biotherapeutics with desired glycan structures to improve their clinical performance. Glycotope’s GEX® technology provides an established expression platform proven for different biopharmaceuticals: Antibodies of different isotypes (e.g., IgG, IgM, IgA) Defucosylated antibodies Bispecific antibodies/fragments (e.g., NK cell/T-cell recruiters) Difficult-to-express…
Novel Single-Use Systems Allow for Faster Powder Transfer and Higher Recovery Rates
Single-use powder containment is vital in today’s safety-focused manufacturing environment. Preventing product from cross-contamination and reducing airborne particulates is a major concern. Understanding how modern bag designs can increase safety and speed is essential for the industry. We looked at the ease of filling along with dispensing times and product loss. ILC Dover’s EZ BioPac® system shows a 71% decrease in filling times when measured against an industry-standard 2D bag. Because of the nonstatic film, we saw an almost 20%…
Amplify Your Titer: Kerry’s AmpliCHO CD Medium
For more than 75 years, Kerry has earned its reputation for reliability and excellence in serving the biotechnology, pharmaceutical, and nutrition markets. We deliver innovative solutions to assist customers increase cell proliferation, extend cell viability, and increase target protein production in biotechnological production systems. Every day we expand our capabilities to meet the changing needs of the biotech market. Kerry’s products have evolved with market trends in cell culture over the past fifty years to fulfill our customers’ requirements. Those…
Gram-Scale Transient Antibody Production and Stable Cell Line Generation Using Flow Electroporation™ Technology
MaxCyte’s delivery platform is a universal, high-performance transfection technology that significantly reduces risk and shortens biotherapeutic development timelines by enabling researchers to Perform early-stage development in the biomanufacturing host cell to ensure identification of high quality, biorelevant candidates Expand the use of transient transfection for faster candidate identification Make rapid and more informed go/no-go decisions through in-depth candidate characterization using transiently produced materials, thus reducing investments associated with stable cell line generation Expedite the transition to biomanufacturing through improved stable…
Consistency in Peptone Manufacturing: Modern Methods Make a Difference
What factors impact peptone variability? Variability can be linked to at least two critical elements: the biological raw materials and the production site. Raw materials can vary by species of animal or plant sourced, type of components (specific animal tissues as opposed to standardized materials), and geographical origins. Feeding practices also can cause variability. Within a single manufacturer, interbatch variability can be linked to the condition and age of a production site, its level of automation and sophistication, optimized production…