MilliporeSigma has developed a new genome editing tool that makes CRISPR more efficient, flexible and specific, giving researchers more experimental options and faster results that can accelerate drug development and access to new therapies. This new technique, called “proxy-CRISPR,” provides access to previously unreachable areas of the genome. Most natural CRISPR systems, found in bacteria, cannot work in human cells without significant re-engineering. However, proxy-CRISPR provides a rapid and simple method to increase their usability without the laborious need to…
Thursday, May 18, 2017 Daily Archives
May 2017 – From the Editor
The first half of 2017 has been leading us to next issue’s focus on the state of the industry. Although we are not officially calling it our “15th anniversary issue,” that of course is what prompts our focus on progress and future projections. By the time you read this, that issue will be on its way to the printer. So here are my thanks, in advance, to those of you who took time with our related survey. We editors look…
May Spotlight
Welcome New Editorial Advisor David Rabuka is global head of research and development in chemical biology at Catalent Biologics, with overall responsibility of overseeing continued research and development of SMARTag technology. He also oversees strategy, resource allocation, and scientific oversight of preclinical and clinical studies. David joined Catalent Biologics following Catalent’s acquisition of Redwood Bioscience Inc., where he was founder, president, and chief scientific officer. David’s scientific areas of expertise include chemical synthesis; drug delivery; translational research; chemistry, manufacturing, and…
Innovating in France’s Auvergne-Rhône-Alpes Region
Lyonbiopole is a French “bio-cluster” based in the Auvergne-RhĂ´ne-Alpes region surrounding Lyon (Figure 1). The cluster supports ambitious projects and companies in the broad health industry, counting more than 200 members including Lyonbiopole’s four founders: Sanofi Pasteur (the vaccines division of multinational pharmaceutical company Sanofi), bioMĂ©rieux (known worldwide for in vitro diagnostics and microbiological testing), Merial (an animal-health company that merged recently with Boehringer Ingelheim), and Becton Dickinson (supplier of flow cytometers, reagents, tools, and services). The region also is…
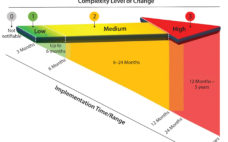
An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…
Opportunities and Challenges in Biosimilar Development
A biosimilar biotherapeutic product is similar (but not identical) in terms of quality, safety, and efficacy to an already licensed reference product. Unlike generic small molecules, it is difficult to standardize such inherently complex products based on complicated manufacturing processes. Table 1 describes the main differences between biosimilar and generic drug molecules. The global biosimilar market is growing rapidly as patents on blockbuster biologic drugs expire (Table 2) and other healthcare sectors focus on reduction of costs. Biologics are among…
Implementing Quality By Design in Analytical Development: A Case Study on the Development of an Anion-Exchange HPLC Method
The concept of quality by design (QbD) initially was outlined in ICH Q8 guidance for drug-product development and later in Q11 for drug-substance development (1, 2). Since then, the QbD concept was further expanded to the development of analytical methods. FDA issued a 2015 guidance on analytical procedures and method validation for drugs and biologics (3). Although the agency did not explicitly state the requirement for implementation of QbD in analytical method development, the concept is embedded in its section…
Scaling Considerations to Maximize the High-Area Advantage
Maximizing filtration-area density is a design strategy to minimize filter footprint and improve filtration process economics. Pleated membrane formats commonly are used to achieve that goal for sterilizing-grade filters operating in dead-end mode (also known as normal-flow filtration). Although high-density pleat geometries increase productivity for a device, such formats can present unique challenges. One of the most common concerns is that pleat formats can introduce flow resistance that impedes a device’s filtration efficiency, particularly for high–pleat-density geometries (1, 2). Filtration…
Current Thinking in Viral Safety: Risk Management Protects Patients
BPI’s editor in chief S. Anne Montgomery recently caught up with long-time editorial advisor Hazel Aranha (purification technologies technology expert for Sartorius Stedim Biotech, North America). They discussed a number of topics related to viral safety. Montgomery: What is the current thinking regarding virus-safety assurance in biopharmaceutical manufacturing? How is the industry preventing viral contamination? Aranha: The “holy grail” of viral safety — absolute freedom from extraneous agents or residual pathogenicity — is a myth. That said, biopharmaceutical products have…