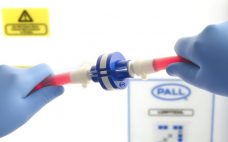
Single-use pre-sterilized systems can potentially offer a higher level of sterility assurance and a lower risk of product contamination by eliminating some of the operational procedures of traditional processing. The importance of this benefit was highlighted by the FDA’s product recalls in 2015 where 78% of recalls were attributed to lack of sterility assurance or to contamination of the drug product, with the primary reason being failure to follow written procedures. A key requirement for multi-component, single-use sterile systems is…
Monday, November 14, 2016 Daily Archives
Ask the Expert: Top Five Considerations in Outsourcing to a Biomanufacturing Partner
with Paul Jorjorian In BPI’s 5 October 2016 webcast, Paul Jorjorian (director of global technology transfer at Patheon) discussed his top five considerations for companies outsourcing to a biomanufacturing partner. Jorjorian’s Presentation Selecting a contract and development manufacturing organization (CDMO) is an important decision for many biopharmaceutical companies. Beyond time and cost, here are some other criteria to consider. The number-one issue is quality. Is your company’s definition of quality the same as a CDMOs — and if…
Ask the Expert: Boosting Profits with Single-Use Powder Transfer
with Chris Rombach Open-suite biomanufacturing is gaining popularity. But it leaves surfaces open to contamination, workers at risk for exposure to airborne particulates, and processes more exposed to product loss due to waste or spillage. Liquid-handling systems are often adapted for dry-powder applications, but flow rates, handling characteristics, and dispensing volumes differ dramatically. ILC Dover’s purpose-built, single-use EZ BioPac system is designed specifically to contain and release dry powders. With single-use bags engineered for the task, it can…
Ask the Expert: BioSC Continuous Chromatography of MAbs, Process Design and Regulatory Considerations
with Vincent Monchois In our September webcast, Vincent Monchois (biopharma strategic project director at Novasep) discussed continuous chromatography using BioSC technology. He included regulatory considerations and the challenges of viral clearance that arise when companies switch from batch to continuous processes. Monchois’s Presentation Users can apply BioSC multicolumn, continuous chromatography to standard capture steps such as ion-exchange or protein A chromatography. The main advantages are continuous operation and full access to the total static capacity of a resin.…