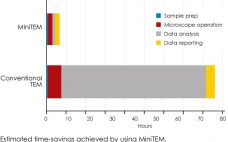
MiniTEMâ„¢ is a low-voltage transmission electron microscope system designed for nanoparticle characterization. The high-quality images it acquires reveal particle morphologies that can be transformed into accurate metrics. A metric for sample purity based on the ratio of the total area of debris to that of intact Adenovirus particles is presented in this study. The automated analysis that MiniTEM offers encompasses a far larger number of particles compared with studies performed manually using conventional electron microscopy, resulting in accurate and statistically…
Monday, October 17, 2016 Daily Archives
A summary of how to develop a cell therapy from concept to market authorization
Cell therapies offer new opportunities for the treatment of disease and injury but considerable infrastructure and a complex range of activities and expertise are required to translate a promising cell therapy product into clinical use. Suppliers, developers, regulators, and care givers need to interact cooperatively in order to bring therapies to patients in the most efficient, safe, and economically viable way and GE Healthcare is working with the Karolinska University Hospital to investigate more effective routes for bringing potential cell…
October Supplement From the Editor
Good things come in small packages — like this issue, for example. Each author brings a critical aspect of regenerative medicine development into perspective, highlighting progress and remaining challenges. I am especially pleased to highlight the current stage of gene therapies. At cell therapy conferences a few years ago, some people insisted to me that quality by design (QbD) would have little to no relevance for these advanced therapies. Speakers were academic and medical researchers or commercial manufacturers of traditional…
Progress Toward Commercial Scale and Efficiency in Cell Therapy Bioprocessing
Regenerative medicine includes both cell and gene therapies. Currently 672 regenerative medicine companies operate around the world, and 20 products have been approved by the US Food and Drug Administration (FDA). Of 631 ongoing clinical trials by the end of 2015 (1), over 40% are in oncology, followed in prominence by cardiovascular and infectious diseases. Here I focus on gene and cell therapy bioprocessing in which the final products delivered to patients are cells. Cell therapies are either autologous (derived…
Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies
The concept of gene therapy is far from new, with initial studies performed over 20 years ago (1). However, in the past few years an explosion of interest in this area has gone beyond initial regenerative approaches using viral vectors. Interest is now moving increasingly into potential use of T cells modified using recombinant viral vectors for immunotherapy applications. Such therapies are based on using either adenoassociated virus (AAV) or lentivirus (1), both vectors being frequently generated through transient expression…
Innovative Downstream Purification Solutions for Viral Vectors: Enabling Platform Approaches to Advance Gene Therapies
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Gene therapies promise versatile treatment options that could revolutionize and transform medicine. As treatment modalities, they offer the possibility of long-term and potentially curative benefits to patients with genetic or acquired diseases. Gene therapies are designed to treat disease by delivering genetic material that encodes a protein with a therapeutic effect into a patient’s cells. It can be used to replace a missing…
Designing the Optimal Manufacturing Strategy for an Adherent Allogeneic Cell Therapy
Cell therapies (CTs) offer potential treatments for a wide range of medical conditions (1–6) by replacing cells, repairing tissues affected by either disease or damage (7), or delivering genetic or molecular agents that promote self-healing (8). CT research and development is continuously growing (9), with increasing numbers of CT candidates reaching phase 3 clinical trials (9–11). Developers aim to make products that can survive in a competitive landscape while complying with stringent regulatory requirements to control the quality and safety…
An Example of Evaluation of protein A Multicolumn Processes for the Capture of Large-Volume, High Concentration Bioreactors, Based on BioSC® Predict Optimization Software
The present paper provides some recent data regarding the optimization of BioSC® processes using three commercially available protein A resins, based on the BioSC® Predict software. A rapid description of the first steps to develop a multi-column process guided by simulation is provided to avoid generating unrealistic conclusions. Other approaches could however be applied depending on the objectives targeted. Examples of multicolumn systems based on three commercially available protein A resins are also provided, for the purification of a 15,000-L…