Thank you for taking the time to view our supplement, which has been driven by our desire to share actionable knowledge in a convenient format as well as to act as a guidepost for the evolution of continuous processing. As you read, we encourage you to ask questions, share your thoughts, or send general input/requests to our team at www.pall.com/ continuous-questions. We hope that this supplement encourages a deeper discussion about continuous bioprocessing technology and the dramatic impact it…
Monday, June 27, 2016 Daily Archives
Authors
The History of Continuous Processing: Why Has the Biopharmaceutical Industry Been So Late to Adopt?
Continuous and semicontinuous manufacturing systems have been used for many years in numerous sectors — including the automotive, food and beverage, oil refining, chemicals, pulp and paper, electronics, metal smelting, steel making, and waste-water treatment industries. Most of these industries are capital intensive and switched to flow manufacturing to increase productivity and flexibility; reduce cycle times, inventory, waste, and costs; and achieve enhanced product quality. Despite the capital intensive nature of drug manufacturing, the biopharmaceutical industry has lagged behind these…
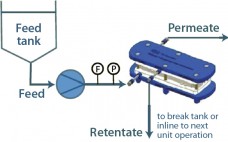
Moving One Unit Operation At a Time Toward Continuous Biomanufacturing: An Overview of Pall Solutions for Integrated Continuous Biopharmaceutical Production
Numerous industries have demonstrated that continuous manufacturing provides significant benefits and advantages, ranging from reduced capital and operating expenses to greater efficiency and product quality and consistency. The conventional approach to biopharmaceutical manufacturing involves the batch performance of a series of unit operations separated by hold steps requiring additional tanks and/or biocontainers. Such an approach fails to maximize facility use, requires large buffer volumes, and results in overall inefficiencies. An integrated, continuous approach to bioprocessing connects each unit operation, minimizing…
Preparing for Continuous Bioprocessing: An Interview with Pall Corporation’s Chief Technology Officer Martin Smith
Martin Smith, PhD, has been with Pall for about nine years and assumed the role of chief technology officer at Pall about 18 months ago. He spoke with Cynthia Challener, PhD, about Pall’s biopharmaceutical business unit and how the company is positioning its technology suite for a continuous process paradigm. Smith: There is no doubt in our minds that we see movement toward continuous bioprocessing. When you look across an array of different industries, the move to continuous or parallel…
Regulating Quality in Continuous Processing
Regardless of the industry and product being manufactured, continuous processing has demonstrated numerous benefits. In addition to smaller manufacturing footprints, reduced material consumption and waste generation, increased efficiencies, and lower capital and operating costs continuous manufacturing typically leads to more consistent processes and product quality. In the pharmaceutical industry, the latter two attributes align perfectly with FDA’s Quality by Design (QbD) and process analytical technology (PAT) initiatives. The challenge is determining how to apply these concepts in practice. Applying the…
Moving Toward a Continuous Future
During the March 2016 BPI West conference in Oakland, CA, BPI publisher Brian Caine and I had an opportunity to meet with Michael Egholm, PhD, Vice President and General manager of Biopharmaceuticals at Pall Life Sciences. We are pleased to be able to share his thoughts about Pall’s support of continuous processing — and the company’s current offerings. Montgomery: Not everyone seems to be defining continuous processing in the same way. What is your general concept of it? Egholm: At…
Using Technology to Overcome Bioprocessing Complexity: Advanced Concentration and Analytical Technologies Accelerate Development and Manufacture of mAbs, Vaccines, and Biosimilars
Unlike chemically synthesized drugs, whose structure is known and reproducible, biological drugs are derived from living cells and are sensitive, complex mixtures requiring cutting-edge biological technologies for their production. The growing importance of biosimilars in recent years is reflected in a corresponding rise in market value. The value of the global biologic therapeutic drug market reached approximately US$230 billion in 2014 and, according to BCC Research, will increase to nearly $390 billion by the end of 2019. This corresponds to…
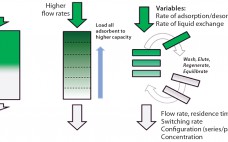
Continuous Chromatography Is Now Possible for Clinical Manufacturing
Intensified and integrated bioprocess technologies are creating a paradigm shift toward more efficient, higher flexibility facilities for biopharmaceutical manufacturing. Continuous technologies that are designed as single-use systems help to greatly facilitate process intensification, delivering further efficiencies with reduced set-up times and elimination of the need for cleaning and cleaning validation. Chromatography is often considered to be a challenging bioprocess step, which has caused great interest in a simplified, safer solution. Continuous multicolumn chromatography using a single-use flow path is an…
Development of a High-Performance, Integrated, and Disposable Clarification Solution for Continuous Bioprocessing
Current bioprocesses combine fed-batch cell culture with batch-wise downstream processing steps. To achieve integrated upstream and downstream continuous manufacturing, the industry has been in need of a continuous cell separation and clarification solution for bioprocess fluids from bioreactors. The Cadence Acoustic Separator from Pall Life Sciences provides this solution, with continuous first-stage clarification without the need for filter media in a scalable, single-use format with no negative impact on product attributes. The Cadence Acoustic Separator delivers cost and time savings…