Here we present a consensus position of the membership of the Bio-Process Systems Alliance (BPSA), the trade organization for the single-use industry based in Washington, DC. BPSA’s membership includes 48 corporate and institutional entities, among them component suppliers, systems integrators, end users, and independent testing laboratories. Consensus within this membership is reached through an official ballot of representative voting members, as provided for in the organization’s by-laws. The position outlined below was approved by such an internal consensus-balloting process. Building…
Tuesday, March 10, 2015 Daily Archives
Extractables Profiles: A Comprehensive Approach Produces Long-Term Results
Biopharmaceutical manufacturers spend years developing and testing new drug and biologic products to ensure their efficacy, safety, and usability for patients. Such knowledge is extremely valuable, but those same principles often get overlooked in selection of packaging and delivery systems. Decisions on packaging and delivery often are made almost as an afterthought. However, issues related to components such as extractables and leachables can affect patient safety and product quality. In addition, a lack of extractables and leachables data in filings…
A Novel Seed-Train Process: Using High-Density Cell Banking, a Disposable Bioreactor, and Perfusion Technologies
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (1). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges. Shake flasks or spinners used in the initial stages require manual manipulations inside a laminar flow hood,…
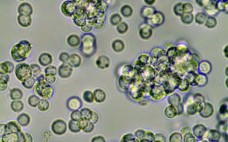
Culturing a Duck ES-Derived Cell Line in Single-Use Bioreactors: A Rapid, Efficient, and Cost-Effective Vaccine Manufacturing System Based on Suspension Culture
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies. In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns…
The New Hybrid: Single-Use Systems Enabled By Process Automation
Much has been written in recent years about the union of single-use systems (SUS) and process automation. These two technology initiatives have been prevalent within biopharmaceutical manufacturing over the past decade and are two of the most predominant advancements in biomanufacturing. A basic survey of industry media and conference topics corroborates that premise (1). However, efforts to combine them remain in the early stages of technological fulfillment, with much work to be done in realizing their synergistic benefits. Here I…
Automation of a Single-Use Final Bulk Filtration Step: Enhancing Operational Flexibility and Facilitating Compliant, Right–First-Time Manufacturing
Single-use technologies have been implemented in biomanufacturing facilities all over the world. Inherently more flexible than stainless steel equipment, single-use technology allows for more rapid technology transfer by minimizing the time it takes to design, purchase, and qualify new capital assets. Rapid turnover between batches is facilitated with no need for protracted clean-in-place (CIP) and steam-in-place (SIP) regimes; the risk of product cross contaminations is reduced because single-use fluid-contact surfaces are never previously exposed to a biopharmaceutical product stream. For…
Measuring Pressure at Very Low Levels with High Accuracy in Single-Use Systems: Improved Performance and Single-Use System Testing
Measuring pressure in single-use systems (SUS) has become an integral part of both upstream and downstream bioprocess operations. Articles have been published on filtration applications (1), and integration into other SUS has been widely adopted. Additionally, information is available on low-pressure applications such as how to prevent overpressurization in single-use bioreactors (2). However, as users and applications both become more sophisticated, improved performance is sought for low-pressure applications (<1 psi) such as in single-use bioreactors. The reasons are two-fold: First,…
Implementing Flexible, Scalable, and Cost-Efficient Bioprocess Platforms: A Proven Project Management Approach
Although significant scientific progress has been made in the biotechnology industry, it has lagged behind other sectors — such as aviation and automotive — in developing, scaling up, and industrializing products coming out of R&D. The goal is to implement robust and reliable manufacturing processes for good manufacturing practice (GMP) market supply cost-effectively. But manufacturing methods for biologics have remained unchanged for decades, with large-scale, capital-intensive stainless steel facilities taking three to five years to build and remaining both energy…
From the Editor – March 2015
In this month’s lineup, you’ll find a common theme of balancing operating costs against company cultures and corporate philosophies. This is far from new: You can’t responsibly run a business without making such calculations! But the value in taking a fresh look at them lies in how we are forced to dust off and reassess some assumptions (even prejudices). It is easy (and all too human) to persist with outmoded assumptions — and we cannot afford to do that given…
Spotlight – March 2015
Niche-Disease: Fanconi Anemia by Cheryl Scott Fanconi anemia (FA) is a rare, genetic blood disorder that causes bone-marrow failure. It prevents bone marrow from producing sufficient new blood cells and/or makes it produce faulty blood cells. Although FA is a blood disorder, it can affect many organs, tissues, and systems. Children who inherit the condition are at higher risk of being born with birth defects. It is a complex and chronic disorder that can be psychologically demanding. FA is not…