Risk assessment is used as a vetting tool to determine areas of influence and uncertainty regarding key factors for critical responses. It helps biopharmaceutical companies build both product and process knowledge during development. Every formulation, unit-operation characterization, and design optimization should begin with risk assessment and ends with an understanding of sensitivity, the quantified influence of key factors, improved control logic, edge-of-failure determination, and a qualified design space.
Quality Risk Management
Quality risk management (QRM) is an essential element in every aspect of drug product and substance development and manufacturing throughout a product's life cycle. According to ICH Q9, QRM is designed to ensure that each drug's critical quality attributes (CQAs) are defined and maintained from phase to phase during product and process development and manufacturing (1). Regarding changes in drug-product formulation, definition, analytical methods, and manufacturing process operations, companies use QRM to understand and manage them, thus ensuring patient safety and drug efficacy. Effective QRM can further secure the high quality of a drug product by providing a proactive means for sponsors to identify and control potential risks to that quality during development and manufacturing.
Risk can be defined two ways: It can encompass a combination of the probability of occurrence of harm and the severity of that harm. And it also can be the potential influence of product and process factors on CQAs with the uncertainty of their influence. The first definition is traditional and takes into account potential failures and adverse events. The second takes into account influence of factors relative to CQAs with how well we understand or know the influence of those factors on a drug product's CQAs. In modern drug development, often the second definition presents a problem in that we don't know what we don't know. As a company completes its product development activities, the risk decreases because accumulated knowledge and understanding of factors associated with unit operations and analytical methods involved increases relative to product acceptance and all associated CQAs.
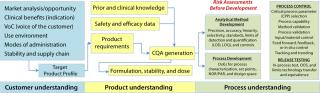
Two key principles apply to QRM (Figure 1): Risk assessment should be based on scientific knowledge associated with product and process understanding; and the level of effort and detail associated with risk assessment/ management should be commensurate with the level of risk being identified and evaluated. Science guides us, and facts/data minimize and help to control risk. A common criticism of risk assessments is that âthey are just opinions and not supported by science.â Focusing and depending on scientific principles, thought experiments, literature research, prior experimental results, and understanding of historical performance brings the best science into a QRM process. The major benefits of QRM and risk assessment come with an improved ability to develop drug products and drug substance while answering these questions: âWhen and where do we need additional development? What kind? And how much?â Development activities reduce potential risks to safety and efficacy and help companies realize the benefits of a drug for its targeted indication.
From QTPP to Acceptance Limits: Systematic drug development and QRM ensure that we maintain a line of sight from our target product profile (TPP) to the quality target product profile (QTPP, Figure 2), through CQA generation to analytical method selection, unit operation characterization, specification and acceptance-criteria limit generation, critical process parameter (CPP) and control selection, and finally method and process validation. ICH harmonized tripartite guidelines detail the pharmaceutical development process and how to set limits (1,2,3,4,5). QRM is linked to product and process CQAs because they constitute the quality attributes of a drug in development.
Risk Assessment Tools
Failure modes and effect analysis (FMEA)
Fault-tree analysis (FTA)
Hazard analysis and critical control points (HACCP)
Hazard operability analysis (HAZOP): preliminary hazard analysis (PHA), statistical/analytical tools, and Pareto and risk-weighted Pareto analysis
Process flow and risk priority numbers (RPNs)
Super fishbone (cause-and-effect diagram with RPN)
Histograms and process capability: control charts; sniper plots; analysis of variance (ANOVA) and hypothesis testing; design of experiments (DoE), product and process)
Variation studies, partition of variation (POV) and restricted maximum likelihood (REML)
QRM Process
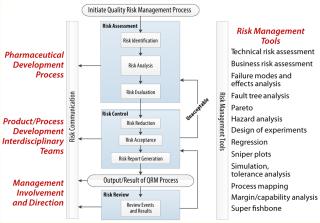
Like any process, QRM includes a series of operations with inputs and outputs (Figure 3). All biopharmaceutical unit operations, formulations, analytical methods, excipients, cell banks, working cell banks, equipment, reagents, chemistry, facilities, and materials are evaluated for the potential influence they can have on drug CQAs and product requirements. Whenever a change is being recommended, a risk assessment should be initiated. Whenever an analytical method is being developed and/or a process is being characterized, a risk assessment should be generated before the study is designed.
Risk Assessment: QRM is linked to product and process CQAs because they are the quality attributes of the drug we wish to assess. Risk assessment includes risk identification, analysis, and evaluation. It is generally a team effort to work through a product/process step-by-step, identifying and evaluating each potential risk. The risk-assessment outcome should be a set of identified and prioritized risks that either require action or can be considered acceptable with an associated rationale. Risk assessments generally should be performed in advance of development or change control rather than used as a justification of changes after the fact.
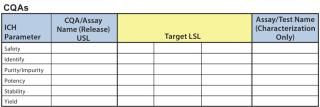
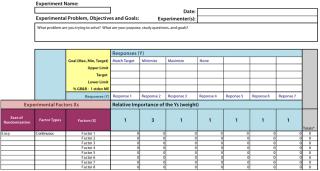
All risk assessments begin with a clear risk question that focuses an assessment team on the specific risk area to be assessed. A list of CQAs should be identified so that there will be a line of sight from those to a given unit operation and/or other drug attribute. Two types of risk assessment templates are commonly used (Figures 4 and 5): QRM high-level risk assessment (such as failure-mode and effects analysis) and a low-level, detailed design-of-experiments (DoE) style factor response matrix.
Risk Control: Risk control (Figure 6) has two potential outcomes. Either we take action to minimize and control risks, or we consider those risks to be acceptable and provide a scientific rationale about why that is the case. Many potential activities can control risk. In general we reduce the severity, lower the probability, and/or improve detectability to lower the risk of a given event occurring. Adding redundancy and/or increasing robustness are also key considerations to design out and control risk. Process analytical technology (PAT), statistical process control (SPC), and associated control logic designs are also important concepts in designing-out risk and designing-in control loops for related inputs and outputs.
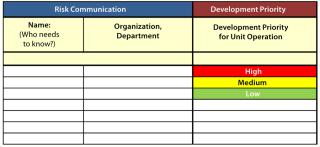
Risk Communication and Review: Once a risk assessment is complete and control actions have been determined, it is critical to the QRM process for the team to communicate those actions to process owners and key stakeholders. Chemistry, manufacturing, and controls (CMC) team leaders, key managers, and development teams will need to know what actions will be required to help minimize identified risks. Clear action owners and implementation timelines and an effective documentation and review process are needed to make all needed and identified changes happen.
QRM Tools: A number of tools typically are used in risk identification, analysis, evaluation, and control (see the âToolsâ box). These tools fall into two categories: risk-assessment and control tools and statistical/analytical tools. The former aid in risk identification, control, and communication (Figure 7)â;. The latter aid in variation analysis, identification of risk areas, and determinatio of the probability and frequency of potential risks. Analytical and quality tools further help companies identify and prioritize risk. Knowing the right tool for the right job is fundamental to proper risk identification and control.
QRM Implementation
Every company involved in drug development needs to take QRM very seriously. You will need an implementation plan with tools, training, and standard operating procedures (SOPs) to build a low-risk development and manufacturing platform. Management involvement is needed to trigger risk assessment generation, form teams, communicate risk-reduction/acceptance actions, and establish a formal review process to measure results and progress.
Clarified roles and responsibilities for QRM within your company also need to be formalized. Risk assessments must be included in development reports, and highlights of those assessments will need to be presented to relevant health authorities in filings and submissions. A clear line of sight from QTPP to lot acceptance and release specifications should be clearly communicated.
Author Details
Thomas A. Little, PhD, is president of Thomas A. Little Consulting, 12401 North Wildflower Lane, Highland, UT 84003; 1-925-285-1847;






Good Information for the QRM