Antibody–drug conjugates (ADCs) are monoclonal antibodies coupled to cytotoxic agents with stable linkers. ADCs travel to target cells, where the antibody binds to its antigen expressed on the cell surface. Upon binding, the full ADC can be internalized by a process called receptor-mediated endocytosis. That process is followed by lysosomal degradation of ADC complexes, which ultimately leads to release of the cytotoxic agent and apoptosis of the target cell. Drugs used in ADCs can be up to a thousand times more potent than current chemotherapeutics (1).
A well-known example of an ADC is brentuximab vedotin, which is often referred to by its trade name, Adcetris (from Seattle Genetics). The US Food and Drug Administration approved this ADC in 2011 for treatment of relapsed or refractory cases of classical Hodgkin lymphoma. It is directed at CD30, which is expressed on the surface of malignant cells, and is linked to three to five units of the antimitotic and antitumor agent, monomethyl auristatin E (MMAE). In clinical trials, Adcetris led to 40% partial and 34% complete remission in patients with refractory Hodgkin lymphoma.Tumor shrinkage also was observed in 94% of patients treated with Adcetris (2).
In 2013, a second ADC with the tradename Kadcyla (from Genentech) was authorized for treatment of HER2-positive metastatic breast cancer. This biotherapeutic consists of the HER2/neu interfering monoclonal antibody, trastuzumab (Herceptin, from Genentech), linked to the cytotoxic agent, mertansine (DM1).
Successful development of an ADC requires optimization of several different components: the structure of an antibody, the potency of a cytotoxic drug, the stability of a linker, the site of conjugation, and stoichiometry of the resulting adducts. Typically, drugs can be linked to antibodies through cysteine or lysine residues, which results in ADCs with a heterogeneous number of drug molecules ranging from zero to 11 per antibody. Variations in drug distribution can have adverse effects on patient health. Antibodies without drugs are ineffective and compete with ADCs for binding to antigen-expressing cells. Products with a high drug-to-antibody ratio (DAR) have less favorable pharmacokinetics, resulting in less favorable in vivo efficacy (3, 4).
A surge in interest and activity in the field of ADCs has led to development of various technologies such as the addition of cysteine residues (5, 6) and incorporation of nonnatural amino acids (7, 8). Another technology targets the carbohydrate moiety of an antibody (9, 10) to generate ADCs with a defined number of drugs per antibody without the prerequisite of genetically modifying the antibody sequence. Unfortunately, the presence of unpaired reactive sulfhydryl groups, use of a huge excess of toxin, or drug loss are some severe drawbacks associated with such chemical modification strategies.
Enzymatic modification of antibodies represents an interesting alternative. Transglutaminases are a family of enzymes capable of forming a covalent bond between the γ-carbonyl amide group of glutamines and the primary amine of lysines. They also can accept substrates other than lysine as the amine donor to modify proteins. For instance, microbial transglutaminase (MGTase) can specifically recognize Gln295 within the heavy chain of unglycosylated IgG1 and be used as a substrate to produce ADCs (11–13). Using MTGase, Dennler and colleagues recently demonstrated that a one-step enzymatic or a two-step chemoenzymatic approach was a reliable strategy to generate homogenous ADCs from native monoclonal antibodies with a defined DAR of 2 (14).
Innate Pharma is a biopharmaceutical company developing first-in-class immunotherapy drugs for cancer and inflammatory diseases. The company is working on a new class of therapeutic agents that consist of monoclonal antibodies aimed at regulatory checkpoints of the innate immune system. As part of its strategy of developing novel therapeutic antibodies, the company has developed a new ADC-coupling technology. Evaluating the stability of those ADC products in aqueous liquid form was paramount to validating the technology concept for clinical applications. This study was designed to monitor the stability of ADC products of SGN30 S/Q aglycosylated mutants coupled to the MMAE toxin by one step (SGN30 S/Q-linker-vcMMAE) or two-step technology (SGN30 S/Q-Linker-Click-Linker-vcMMAE). The following results compare the aggregation propensity and the physical and chemical stability of these ADC products with Adcetris. Data obtained with ProteoStat dye (from Enzo Life Sciences) were correlated with results obtained with other analytical methods, including size- exclusion–high-performance liquid chromatography (SE-HPLC) and liquid chromatography–mass spectrometry (LC-MS).
Materials
In this study, we monitored the stability of ADC products of SGN30 aglycosylated mutants S and Q coupled to the MMAE toxin by two-steps technology (SGN30 S/Q-Linker-Click-Linker-vcMMAE) and one-step technology (SGN30 S/Q-linker-vcMMAE) as liquid aqueous form. Stability of the ADC products shown in Table 1 was evaluated to validate the technology platform for clinical applications.
We compared the stability of these ADC products with that of Adcetris (brentuximab vedotin), which was used as a reference control. To provide comparable results, all ADC products were formulated in the aqueous formulation of Adcetris with an upgraded polysorbate 80 concentration from 0.2 mg/mL (original Adcetris formulation) to 1 mg/mL (Table 2). Diluent, Adcetris DIL, was an aqueous solution formulated like Adcetris, with a 1-mg/mL concentration of polysorbate 80 and without active product (Table 3).
ProteoStat protein aggregation standards (IgG) (ENZ-51039- KP002), ProteoStat protein aggregation assay (ENZ-51023- KP002), and ProteoStat thermal shift stability assay (ENZ-51027-K400) came from Enzo Life Sciences (Farmingdale, NY).
Methods
Preparation of Protein Aggregation Standards: Sheep IgG (4 mg/mL) were incubated in low pH buffer (0.2M glycine-HCl, pH 2.5) at 50 °C overnight with shaking at 400 rpm in a shaking heat block for 20 hours. We diluted 100% IgG aggregates with native sheep IgG monomers to a final concentration of 1 mg/mL, consisting of 12.5%, 6.25%, 3.13%, 1.56%, 0.78%, 0.39%, 0.20%, and 0% aggregated IgG.
Aggregation Assay: We used ProteoStat protein aggregation assay to quantify and compare the soluble, nonsoluble, and noncovalent aggregate levels between the ADC products. ADC and protein aggregation standards were mixed with ProteoStat detection reagent (final dye dilution of 1:2,000). After incubating solutions for 15 minutes in the dark at room temperature, we recorded the fluorescence levels using excitation/ emission setting of 550/600 nm.
Thermal Shift Stability Assay: The temperature of aggregation (TAgg) was measured using ProteoStat thermal shift stability assay. ADC products with a 5 mg/mL initial concentration were diluted five times in Adcetris DIL to obtain sample volumes of 180 μL at a 1.0-mg/mL concentration. We ran each 50-μL sample in triplicate and diluted 1,000× ProteoStat thermal shift detection reagent with 1× ProteoStat thermal shift diluent (final dye dilution of 2×).
LS55 spectrofluorometer (Perkin Elmer) fluorescence was read and recorded continuously while the temperature was ramping from 40 °C to 100 °C by 0.5 °C step (5-second equilibrium step duration). We determined TAgg as the maximum point in the first derivative (slope) of the fluorescence curve (dF/dT°).
SE-HPLC: We analyzed the ADC products and Adcetris using ultraperformance liquid chromatography (UPLC) (Acquity, from Waters) with a BEH200 SEC 1.7 μm column. Single runs were performed for each sample and for each condition.
LC–MS: ADC products were eluted on a C18 RP-HPLC column and identified using double detection by UV (DAD, from Agilent Technologies) and electrospray ionization–quadrupole time-of-flight mass spectrometry (ESI-Q-TOF) (microTOF QII, Bruker). We analyzed the ADC products with the intact antibody method (C18 PLRP-S polymeric column). Because the Adcetris product is not compatible with the intact-antibody method (the antibody is partially reduced), we analyzed the products using the reduced-antibody method (C18 Aeris column) after N-deglycosylation with PNGase F. A standard deviation of ±0.1 was established with this technique.
Sampling for Physical Stability Study: Freeze–thaw (F/T) and shaking stresses were performed on ADC products and Adcetris at their original concentration (5 mg/mL). Three conditions were chosen to test physical stability: no stress, 3F/T (three freeze–thaw cycles), and shaken. A volume of 315 μL (equivalent to 1.575 mg) per product and per condition were required to perform the aggregation assay (300 μL), SE-HPLC (10 μL), and LC-MS (5 μL).
Sampling for Chemical Stability Study: The 5 °C and 40 °C storage conditions were studied on ADC products and Adcetris at their original concentration (5 mg/mL). Five time-points were used to test chemical stability: T0, one week, four weeks, 10 weeks and 24 weeks. At T0, we tested the 5 °C storage condition. Product quantities required for SE-HPLC (10 μL) and LC-MS (5 μL) testing were already included in the no-stress condition of the physical stability study. A volume of 15 μL (equivalent to 0.125 mg) per product, per condition, and per time-point was required to perform SE-HPLC (10 μL) and LC-MS (5 μL) analysis.
Results
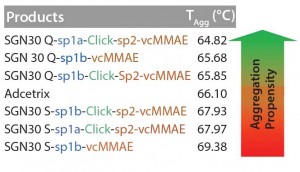
Aggregation Propensity: Measurement of aggregation temperature (TAgg) allows classification of ADC products according to their propensity for aggregation. Low TAgg values correlate with a higher aggregation propensity. Using the ProteoStat dye for TAgg determination, we showed that SGN30 Q products had a higher aggregation propensity than did SGN30 S products (Figure 1). SGN30 Q-sp1a-Click-sp2- vcMMAE demonstrated a significantly higher aggregation propensity (TAgg = 64.82 °C) than did SGN30 Q-sp1b-vcMMAE (TAgg = 65.68 °C) and SGN30 Q-sp1b-Click-sp2-vc MMAE (TAgg = 65.85 °C) (Figure 1). The standard deviation between those last two ADC products was ±0.3 °C, and the difference in TAgg was not significant. For the same reason, the difference in TAgg was also not significant when comparing SGN30 S-sp1a-Click-sp2-vcMMAE (TAgg = 67.97 °C) and SGN30 S-sp1b-Click-sp2-vc MMAE (TAgg = 67.93 °C) (Figure 1). But SGN30 S-sp1b-vcMMAE had a significantly higher TAggthan any other ADC product (TAgg = 69.38 °C) (Figure 1). Taking into account the standard deviation of TAgg measurement (±0.3 °C), Adcetris has a similar propensity for aggregation (TAgg = 66.10 °C) than did the SGN30 Q candidate with the highest TAgg value, SGN30 Q-sp1b- Click-sp2-vc MMAE (TAgg = 65.85 °C).
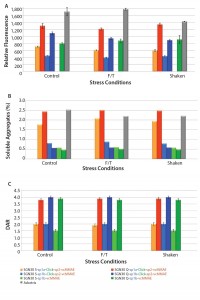
Figure 2: Aggregation and drug–antibody ratio (DAR) in ADC products upon induction of physical stress; resistance to three freeze–thaw cycles (F/T) and shaking was determined using ProteoStat dye (a), SE-HPLC (b), and LC-MS (c) and compared with control.
Physical Stability: We chose three conditions to test the physical stability of the ADC products: no stress, 3 F/T (three freeze–thaw cycles), and shaken. The dye demonstrated that freeze–thaw cycles and shaking stresses had no significant effect on the amount of protein aggregates in solution when compared with control (Figure 2a). We used SE-HPLC to measure the levels of soluble and covalent/noncovalent aggregates that could be generated following those stresses. Shaking had no effect on the percentages of soluble aggregates (Figure 2b). Finally, LC–MS analysis showed that freeze–thaw cycles and shaking did not significantly alter the integrity of the molecular structures of these ADC products and that the drug-to-antibody ratio (DAR) remained the same regardless of the induced stress (Figure 2c).
Chemical Stability: ADC products and Adcetris were stored for six months in conditions of normal storage (5 °C) or accelerated degradation (40 °C). SE-HPLC and LC-MS monitored their chemical stability after one week, four weeks, 10 weeks, and 24 weeks.
SE-HPLC showed a slight increase in the percentage of soluble aggregates after 24 weeks at 5 °C with SGN30 Q-sp1b-vcMMAE (from 0.46% to 3.96%) and SGN30 S-sp1b-vcMMAE (from 0.57% to 3.14%). The other ADC products were not affected by this treatment (Figure 3a). But storage at 40 °C led to a more notable increase in the percentage of soluble aggregates, especially for SGN30 Q products that are coupled with four MMAE lipophilic toxins (up to 23.58% after six months) as compared with SGN30 S products with only two (Figure 3b). For both SGN30 Q and S products, those with a Sp1a lipophilic linker showed the highest increase in the percentage of soluble aggregates over time (from 2.43% to 15.99% for SGN30 Q-sp1a-Click-sp2-vcMMAE; and from 1.77% to 4.60% for SGN30 S-sp1a-Click-sp2-vcMMAE over 10 weeks) (Figure 3b). Interestingly, Adcetris had one of the highest increases in soluble aggregates over time (from 2.53% to 16.59% over 24 weeks). Finally, the DAR value remained the same over six months regardless of the storage temperature, as demonstrated by LC-MS (Figures 3c and 3d).
Discussion
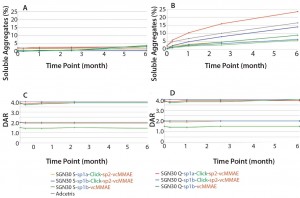
Figure 3: Measurement of aggregation, protein fragments, and drug-to-antibody ratio (DAR) in
ADC products stored at 5 °C or 40 °C over a period of six months; stability at 5 °C (a and c) and 40 °C
(B and D) was determined using SE-HPLC (a and b) and LC-MS (c and d).
After measuring the TAgg of ADC products, results confirm the initial hypothesis that the greater the number of MMAE toxins coupled to the monoclonal antibody, the higher the aggregation propensity. SGN30 Q products had a higher aggregation propensity than did SGN30 S products because of differences in the lipophilic nature of the MMAE toxin and DAR values of SGN30 Q and S products. SGN30 Q and S have a respective DAR value of 4 and 2, which makes SGN30 Q products more lipophilic and therefore more prone to aggregation.
Moreover, SGN30 S-sp1b-vcMMAE showed a significantly higher TAgg than any other ADC product tested. We used a one-step coupling reaction to generate this ADC, and because of this, the target DAR value of 2 could not be reached. The actual DAR value for this ADC is 1.5, indicating that it is less lipophilic because it contains fewer MMAE toxin molecules. Consequently, it is less prone to aggregation than other SGN30 S products with a DAR value of 2.
This assay also validated the hypothesis that a more lipophilic linker between the monoclonal antibody and the toxin leads to a higher aggregation propensity. Indeed, SGN30 Q-sp1a-Click-sp2-vcMMAE, containing the most lipophilic linker, Sp1a, demonstrated a significantly higher aggregation propensity than other ADC products. However, that is true only for SGN30 Q products that are coupled with four linkers to four MMAE molecules. Such variation is not significant for SGN30 S products, because they are coupled with only two linkers to two MMAE molecules.
Based solely on this technique, SGN30 Q-sp1b-Click-sp2-vcMMAE and SGN30 S-sp1b-Click-sp2- vcMMAE were deemed to be the most stable Q and S products. Adcetris contains three to five molecules of MMAE toxins per antibody, whereas SGN30 S products contain only two; hence, a higher position of Adcetris in the aggregation propensity ladder than SGN30 S products.
In addition, SGN30 Q and S products are unglycosylated mutants, but Adcetris is glycosylated. Glycosylation stabilizes proteins and protects against aggregation, which can explain why Adcetris is situated lower on the aggregation propensity ladder than SGN30 Q products despite having in average a similar number of MMAE toxins per antibody. However, Adcetris had one of the highest increases in percentage of soluble aggregates observed in SE-HPLC over time and could be considered as the second worst candidate with SGN30 Q-sp1a-Click-sp2-vcMMAE.
Physical stress tests had little to no effect on Innate Pharma’s ADC products. Levels of aggregation and DAR values remained the same, regardless of induced stress. Both the protein element (from Innate Pharma) and the link with the MMAE toxin were stable when such physical pressures were applied. Results obtained with the aggregation assay correlated with data obtained from SE-HPLC and LC-MS analysis.
Under normal storage conditions, integrity of the protein element and linkers used in Innate Pharma’s antibodies remained uncompromised over a period of six months. Both the ADC products and the Adcetris product are stable over six months when stored at 5 °C. Conversely, the ADC products showed a significant increase in aggregation after storage conditions of accelerated degradation, especially for SGN30 Q products and Adcetris. Our observations matched with the aggregation propensity predictions obtained using the thermal shift stability assay: The aggregation propensity increased with the number of MMAE toxins coupled to the antibody and the lipophilic character of the linker. Furthermore, the DAR value remained the same throughout the experiment, suggesting that the linkers (developed by Innate Pharma) are stable and robust even when stored at 40 °C.
Comparable Dye Method
We determined the stability of ADC products and evaluated the suitability of the ProteoStat dye for analyzing them. Combining aggregation propensity with physical and chemical stability studies highlighted several important facts about Innate Pharma’s ADC coupling technology. Notably, the stability of an ADC depends largely on the number of toxins conjugated to the antibody and the lipophilic nature of linkers used to conjugate those toxins. Applying the dye resulted in the successful prediction of the propensity for aggregation of ADC products, thereby validating the coupling technology as an ideal platform for development of stable, robust ADC products.
References
1 Sievers EL, Senter PD, et al. Antibody– Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 64, 2013: 15–29.
2 Younes A, et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients with Relapsed or Refractory Hodgkin’s Lymphoma. J. Clin. Oncol. 30(18) 2012: 2183– 2189.
3 Hamblett KJ, et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 10(20) 2004: 7063–7070.
4 Boswell CA, et al. Impact of Drug Conjugation on Pharmacokinetics and Tissue Distribution of Anti-STEAP1 Antibody–Drug Conjugates in Rats. Bioconjugate Chem. 22(10) 2011: 1994–2004.
5 Junutula J, et al. Site-Specific Conjugation of a Cytotoxic Drug to An Antibody Improves the Therapeutic Index. Nat. Biotechnol. 26(8) 2008: 925–932.
6 Shen B, et al. Conjugation Site Modulates the In Vivo Stability and Therapeutic Activity of Antibody–Drug Conjugates. Nat. Biotechnol. 30(2) 2012: 184– 189.
7 Axup J, et al. Synthesis of Site-Specific Antibody–Drug Conjugates Using Unnatural Amino Acids. Proc. Natl. Acad. Sci. 109(40) 2012: 16101–16106.
8 Xiao H, et al. Genetic Incorporation of Multiple Unnatural Amino Acids into Proteins in Mammalian Cells. Angew. Chem. Int. Ed. Engl. 52(52), 2013: 14080–14083.
9 Zuberbuhler K, et al. Fucose-Specific Conjugation of Hydrazide Derivatives to a Vascular-Targeting Monoclonal Antibody in IgG Format. Chem. Commun. 48(56) 2012: 7100–7102.
10 Okeley NM, et al. Metabolic Engineering of Monoclonal Antibody Carbohydrates for Antibody–Drug Conjugation. Bioconjug. Chem. 24(10) 2013: 1650–1655.
11 Jeger S, et al. Site-Specific and Stoichiometric Modification of Antibodies By Bacterial Transglutaminase. Angew. Chem. Int. Ed. Engl. 49(51) 2010: 9995–9997.
12 Mindt T, et al. Modification of Different IgG1 Antibodies Via Glutamine and Lysine Using Bacterial and Human Tissue Transglutaminase. Bioconjugate Chem. 19(1) 2008: 271–278.
13 Dennler P, et al. Enzymatic Antibody Modification By Bacterial Transglutaminase in Antibody–Drug Conjugates. Ducry L, Ed. Humana Press: New York, NY, 2013; 205.
14 Dennler P, et al. Transglutaminase- Based Chemo-Enzymatic Conjugation Approach Yields Homogeneous Antibody– Drug Conjugates. Bioconjug. Chem. 25(3) 2014: 569–578.
Nicolas Schneider is a pharmacist, pharmaceutical operations, at Innate Pharma, Morgan Mathieu is an applications scientist at Enzo Life Sciences. Sandra Savard-Chambard Gas is a technician, QC and bioanalytical development, Angélique Boedec Herbette is a scientist, QC and bioanalytical development, Hélène Rispaud is an engineer, QC and bioanalytical development, Naouel Lovera is a technician, QC and analytical development), Delphine Bregeon is an R&D scientist, pharmaceutical operations, Agnès Represa is manager, QC and bioanalytical development, and Christian Belmant is associate director, pharmaceutical operations, all with Innate Pharma. Courtney Noah is senior marketing manager at Enzo Life Sciences; cnoah@enzolifesciences.com.