Given the current drive to reduce the cost of manufacturing for biological therapeutics, it is incumbent upon chromatographers and process engineers to streamline the manufacturing process wherever possible. As a result of this need, the demands on downstream unit operations have increased to the point where a single processing step is expected to accomplish a multitude of purification objectives. Introduction Anion exchange chromatography is a widely used technique for protein capture or impurity removal. Typically, anion exchange resins with quaternary…
Friday, August 15, 2014 Daily Archives
Purification of Influenza Virus By Ion-Exchange Chromatography on Mustang® QXT Membranes
Membrane chromatography is an effective alternative to conventional beaded sorbents for the purification of large virus molecules or viral-like particles. Membranes have an open pore structure that allows fast kinetics and direct access to ion-exchange binding sites, with flow rates that are orders of magnitude faster than conventional columns. They provide much higher binding capacities than beaded sorbents for large molecules limited by mass transfer. Pall Mustang ion-exchange membranes are scalable membrane chromatography devices used in various therapeutic, vaccine, and…
Successful Filtration Scale-Up for an Automated, Single-Use, Final Bulk-Fill System
This application note describes a case study of the correct sizing for a filter involved in an automated final bulk-fill system for a biopharmaceutical product. It demonstrates how customer batch-processing requirements can be attained with a robust experimental design. Scale-Up Methodology Figure 1 shows a process for successful scale-up. Once the desired process has been defined, filter performance can be determined by either constant flow or constant pressure testing on disc formats. The data can be scaled-up easily to determine…
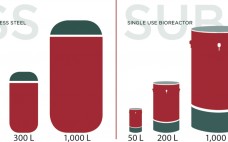
Novel Single-Use Sensors for Biopharmaceutical Applications
The increasing adoption of single-use technology in biopharmaceutical, vaccine, and cell therapy production is one indication that such technology has moved far beyond its novelty stage. Arguably, this is the preferred technology of newly developed processes. To support the continued growth of single-use systems in the biopharmaceutical industry, the ability to measure critical process parameters with single-use sensors is required. Several key single-use sensing technologies are described, including pressure, temperature, UV absorbance, and conductivity. The technology behind each is explored…
Protein A Chromatography with High-Titer Feedstocks
Protein A chromatography has become a widely used platform in monoclonal antibody (MAb) purification. We describe the impact of higher MAb loadings on aggregation and ligand leaching during protein A chromatography, as well as the benefits of high-capacity Protein A resins for high-titer feedstocks. Today, high MAb titers achieved through advanced upstream processing have become a challenge for downstream processing, especially with regards to the expensive protein A capturing step. TOYOPEARL AF-rProtein A HC-650F provides superior MAb capacity and beneficial…
Process Innovation Leads to More Affordable Biosimilars
Biosimilars are set to dramatically improve how millions of people access expensive, complex, life-saving treatments by providing equally effective alternatives to innovator products while lessening the cost burden (>30%). Therapeutic Proteins International, LLC (TPI) is a fully integrated manufacturer of biosimilar recombinant protein products that has reinvented conventional approaches to biologic drug manufacturing. Powerful Science Shouldn’t Be Complicated TPI has reinvented the process used to manufacture biological drugs making it simpler by leveraging superior technology and rigorous scientific methods to…
Who We Are
Major Markets AbbVie Contract Manufacturing serves domestic and global companies seeking to outsource biologics, potent capabilities, drug products, fermentation, prefilled syringe manufacturing, and HME, as well as businesses looking to procure APIs. Outsourcing clients range from mid-size to large pharmaceutical and biotech companies. Services Offered Biologics: AbbVie is a leading biopharmaceutical developer and manufacturer, with a scientific team that offers expertise in process optimization, analytical characterization, and current good manufacturing practice (CGMP) manufacturing to support full development and commercialization of…
Aeras: Leveraging Capabilities to Meet Vaccine Development Challenges
Aeras is a nonprofit biotech focused on the development of TB vaccines. Together with our global research and development partners, we have played an integral role in developing approximately half of all TB vaccine candidates in clinical development around the world. TB is one of the most deadly infectious diseases in the world. It spreads through the air like the common cold. A TB patient with active disease can infect up to 15 people simply by coughing, sneezing, or talking.…
Your Partner from Concept to Commercial
Avid Bioservices is a contract manufacturing organization (CMO) specializing in mammalian cell culture process development and current good manufacturing practice (CGMP) production of clinical- and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to your success, our team is constantly striving to build partnerships that extend well beyond the delivery of product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements. Established CMO with Proven Capabilities nearly two decades of…
Producing Value with Boehringer Ingelheim BioXcellence™
Boehringer Ingelheim BioXcellenceâ„¢ is your dedicated biopharmaceutical contract manufacturer. As a leading biopharmaceutical contract manufacturer with more than 35 years of experience, it has brought 22 biopharmaceutical products to market. Boehringer Ingelheim BioXcellenceâ„¢ offers tailor-made contract development and manufacturing services to the biopharmaceutical industry, providing the entire production technology chain from DNA to fill and finish under one roof at its facilities in Biberach (Germany), Vienna (Austria), Fremont (USA), and Shanghai (China). Boehringer Ingelheim BioXcellenceâ„¢ can secure product supply throughout…