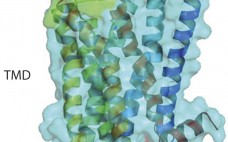
G -protein coupled receptors (GPCRs) represent a target superfamily linked to many disorders across all therapeutic areas. Although this target class has been historically treated by small molecules and peptides, antibodies can offer a number of advantages over such molecules by virtue of their specificity, dosing frequency, and restricted penetration. They also can provide other functional effects specifically mediated by the Fc region (ADCC and CDC) as well as different modalities such as those offered by bispecific and antibody drug…
Sunday, June 1, 2014 Daily Archives
Site-Specific Characterization of Glycosylation on Protein Drugs
A large proportion of biotherapeutic products are glycoproteins. These include erythropoietin and other cytokines, antibodies, glycosyltransferases, and glycosidases, which together generate billions of dollars in sales worldwide. Such drugs are inherently complex. As new treatments emerge and biosimilars are evaluated, the need to better understand their molecular structures is more acute than ever. Therapeutic glycoproteins are typically produced as recombinant products in cell culture systems. Glycosylation is of major importance during development of these drugs because their glycan chains markedly…
Process Challenges of Antibody–Drug Conjugates
With two products now on the market, and a host of others in clinical trials, antibody-drug conjugates (ADCs) are slowly becoming a big business. Designed to deliver extremely active cytotoxic drugs that are otherwise undosable, they take advantage of the targeting ability of a specifically designed monoclonal antibody (MAb) to “shield” a highly potent API (HPAPI) as it travels through a patient’s bloodstream after administration. Once the antibody reaches its target on the cancer cell, it will release the payload,…
The Rise of Biopharmaceutical Outsourcing to Indian CDMOs
India is becoming an increasingly attractive destination for outsourcing biotechnology services by global biopharmaceutical companies. As “Big Pharma” continues on its path of finding ways to lower costs for development and manufacturing of biopharmaceuticals, Indian contract development and manufacturing organizations (CDMOs) are being viewed as capable and beneficial service providers that possess the necessary technical expertise and regulatory-compliant facilities. According to its 11th annual report on biopharmaceutical manufacturing capacity and production, BioPlan Associates ranked India fourth in the world as…
Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 2 — Application
Here we apply our approach to validation of animal cell culture process changes using qualified, scale-down bioreactors. As described in Part 1 (including Table 1, Figures 1 and 2, and References 1–23), the goal is to facilitate implementation for the benefit of both the patients and industry. “Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell–Derived Products, Part 1 — Concept” appears on pages 38–45 of BioProcess International’s May 2014 issue. Process changes often entail validation,…
Enabling Greater Process Control and Higher Protein Titers: Advances in Downstream Single-Use Technologies
Downstream protein purification (the stage in which a protein is isolated and purified) is one of the last steps in biotherapeutic manufacturing. Single-use technologies are an increasingly popular choice for both upstream and downstream bioprocessing because they offer significant benefits over traditional multiuse manufacturing systems. Single-use technologies also provide an array of logistical benefits, including reduced costs, minimized risk of cross-contamination, and improved operational efficiency (1). Challenges remain, however, in designing a complete, streamlined, single-use process for downstream protein purification.…
Rapid Development and Scale-Up Through Strategic Partnership: Case Study of an Integrated Approach to Cell-Line and Process Development for Therapeutic Antibodies
Over the past decade, monoclonal antibodies have become mainstream therapeutics for treating a broad range of conditions from autoimmune disorders to cancer. Part of this evolution is increasing time and cost pressure on biopharmaceutical companies to bring new drugs to market 1, 2. Additionally, companies now routinely engineer and screen molecules for developability and manufacturability during discovery before selecting a final candidate molecule. The biosimilar development paradigm also demands significantly more bioanalytical analysis during initial cell-line and process development. Thus,…
One Billion Mesenchymal Stem Cells in an Eppendorf BioBLU 5c Single-Use Bioreactor at 3.75-L Scale
For BPI’s inaugural “Ask the Expert” webcast, Ma Sha (Eppendorf’s director of technical applications) fielded questions related to his upcoming poster presentation at IBC’s Single-Use Applications for Biopharmaceutical Manufacturing in Boston this month: “One Billion Mesenchymal Stem Cells in Eppendorf BioBLU 5c Single-Use Bioreactor 3.75-L Scale”. Eppendorf R&D Labs is formerly New Brunswick Scientific, which was acquired by Eppendorf in 2007. Sha’s Presentation Our focus recently had been large-scale stem-cell applications in bioreactors. We chose to work on mesenchymal stem…
Transformative Healthcare: The National Biomarker Development Alliance
A newly launched independent, nonprofit organization — the National Biomarker Development Alliance (NBDA) — will broadly engage leaders in industry, academia, patient groups, and government from across the United States. It was announced on 13 January 2013 at the National Press Club by the Research Collaboratory (RCASU) at Arizona State University (ASU). The mission of the NBDA is to address the complex and urgent challenge of creating standards needed for end-to-end, systems-based biomarker research and development (R&D). The alliance is…
Strategies to Mitigate Technology Transfer and Clinical Manufacturing Risks: Downstream Purification Case Studies
Since the early 1980s, biotechnology products have been a fast-growing sector. They now occupy a significant portion of biologic drugs approved by regulatory authorities around the world every year. Among the approved biologic drug products, as well as those still in clinical testing, many are manufactured by contract manufacturing organizations (CMOs). Sponsor companies often transfer their developed process and process knowledge to CMOs for manufacturing of materials for toxicology and clinical studies. Drug Product Development Figure 1 illustrates the usual…