Cell therapy is offering a promising future in medical advances. Although multilayer trays are suitable for R&D and preclinical cell amplification, they cannot support large-scale industrial production. A successful transition from laboratory scale to an efficient and robust process based on good manufacturing practice (GMP) is key.
The Integrity® Xpansion™ multiplate bioreactors have been specifically designed to enable an easy transfer from existing multiple-tray stacks processes by offering the same cell growth environment on two-dimensional (2D) hydrophylized polystyrene (PS) plates in a fully closed system. To make the bioreactor compact, the headspace between each plate has been reduced to a minimum (1.3 mm). Gas transfer is made through a semipermeable silicone tubing mounted in the central column. A magnetic stirrer at the bottom of the bioreactor ensures a homogenized distribution of gas. Preserving the cell culture environment is critical as small variations in physicochemical parameters such as surface characteristics can heavily impact stem cell growth and behavior. Additionally, critical cell culture parameters such as pH and DO are controlled.
Case StudyPromethera Biosciences (www.promethera.com) is developing cell therapies to treat several liver genetic metabolic diseases such as the Crigler-Najjar syndrome. Human heterologous adult liver progenitors cells (HHALPCs) were initially cultivated in 2D standard cultivation devices. This study demonstrates the feasibility to cultivate HHALPCs in Xpansion bioreactors with 10, 50, and 180 plates (equivalent to 110.160 cm2 cell culture surface).
Results: A process was transferred from multitray stack to Xpansion 10 to prove the feasibility of stem cell growth in the Xpansion multiplate bioreactor and to optimize cell culture parameters. All runs achieved results similar to their control in terms of cell density, homogenous distribution, viability, and morphology. As Table 1 shows, additional quality control (QC) analysis revealed that cell characteristics were maintained (identity/purity/potency).
Table 1:Scale-Up from the Xpansion 10 to the Xpansion 180: Cultures were directly transferred from the Xpansion 10 bioreactor to larger-scale Xpansion 50 and 180 bioreactors, where cells reached similar levels of growth and confluence (Table 1). Further analysis of the cultures at all scales showed compliancy with the QC specifications. To keep the process within a closed system, cells harvested from the Xpansion 180 bioreactor were directly centrifuged. The in-line continuous centrifugation step achieved 80% yields while maintaining identical cell characteristics (Table 1).
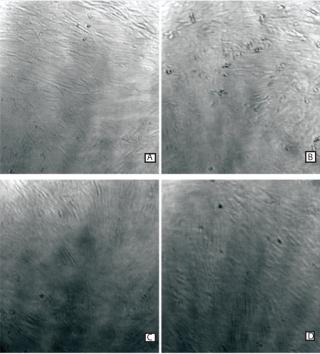
Cell Observation Using the iLine Holographic Microscope: The iLine holographic microscope from Ovizio (www.ovizio.com) has specifically been designed for cell observation on the Xpansion bioreactor. The microscope software enables an automatic cell counting of the cell confluence. Pictures were taken at the end of a culture in the Xpansion 180 bioreactor and (Figure 1). Cell confluence assessment through DDHM microscope is a key element for defining cell harvest time given that cell confluence levels are critical to guarantee cell characteristics.
Integrity Xpansion multiplate bioreactors demonstrated their efficiency for the growth of mesenchymal stem cells at large scale while keeping cell therapeutic potency. Use of the iLine microscope enabled recording of the culture’s evolution along with a robust process control system: a sampling port that can be used for dosing of nutrients, growth factors, and so on; on-line pH and dissolved oxygen (DO) tracking; and off-line microscopic observations.
The Xpansion 10 bioreactor proved to be a useful tool for determining optimal cell culture parameters. Actually, several runs could be performed using this scale-down model, while sparing time and money, and extrapolating the cell behavior and the pH and DO trends in the Xpansion 50 and the Xpansion 180 bioreactors. The new Xpansion bioreactor offers a valuable technology for large-scale production while meeting GMP compliancy. Moreover in line centrifugation step guarantees a closed manufacturing process, from seeding to freezing
Author Details
Matthieu Egloff is global product manager of cell therapy at ATMI, rue de Ransbeek 310, 1120 Bruxelles, Belgium; 32-2-264-1868;
REFERENCES
235.