Adherent cell processes for production of adenoviruses often involve 2D vessels that require many manual operations and large areas of cleanroom space, or microcarrier cultures that require complex cell transfer steps and a very high level of expertise. To maximize surface area within a compact space, Integrity® iCELLis® bioreactors contain macrocarriers trapped in a fixed-bed, providing ≤500 m2 of 3D growth surface area in only 25 L of fixed-bed. The iCELLis technology can be used at small scales (iCELLis nano bioreactor, starting at 0.53 m2) and large scales (iCELLis500 bioreactor, ≤500 m2 growth surface) with straightforward process scale-up, easy single-use operation, and minimal space requirements.
Transfer and Scale-Up of a HEK293 Cell Culture Process for Adenovirus ProductionAn existing process using HEK293 cells for production of an adenovirus was first transferred from multitray systems to an iCELLis nanobioreactor (0.53m2) by keeping equivalent cell culture parameters. Additional experiments were performed with lower cell-seeding densities to reduce the number of preculture steps at large scale. The following parameters were also optimized for cell growth and virus productivity: fixed-bed height, compaction of carriers inside the fixed-bed, and linear velocity of medium through the fixed-bed (cm/s).
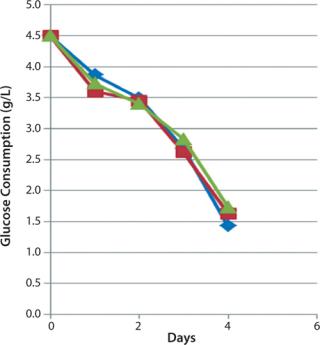
Industrial Scale-UpAfter determining optimal parameters at small scale, HEK293 cell culture batches were performed in duplicate with small- and large-scale bioreactors. Inoculation density, medium volume ratios, culture duration, pH, dissolved oxygen, and temperature set points were kept identical at both scales. Consistent cell density results between 2.8 and 3.8 × 105 cells/cm2 (3.5 × 109 cells/bioreactor) were achieved at small scale, and between 2.7 and 3.4 × 105 cells/cm2 (total of 4.1 × 1011 cells/bioreactor) at large scale. Analysis of glucose and lactate (Figures 1A and 1B) at both scales compared with a five-tray Cell Factory control indicated that cell metabolism was comparable between small-and large-scale iCELLis bioreactors and the standard 2D process.
This study demonstrates that the fixed-bed design of the iCELLis bioreactor enables high cell densities to be achieved and maintained in both small and large bioreactor volumes. The iCELLis bioreactor can be inoculated at a very low cell density, leading to a dramatic simplification of seed-train operations and a significant reduction of development timelines.
In conclusion, large biomass amplification and excellent virus productivity, combined with the advantages of a fully closed disposable system with low shear stress, make the iCELLis fixed-bed bioreactor a simple and straightforward solution for industrial virus production.
Author Details
Shane Knowles is a senior applications specialist at ATMI, rue de Ransbeek 310, 1120 Bruxelles, Belgium; 32-2-264-1885;