The capture step in the downstream processing of monoclonal antibodies (MAbs) is often the bottleneck and the most expensive step due to the use of protein A media. In recent years, several new-generation protein A media have been launched on the market claiming improved MAb capture, or improved resistance to cleaning agents.
AbSolute® High Cap, the new revolutionary protein A media by Novasep, maintains excellent DBCs at low and high velocities, therefore, providing substantially improved productivity and strongly reduced costs compared with all existing protein A media.
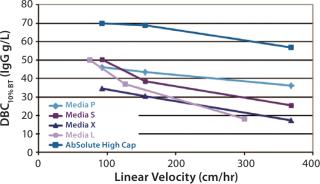
Outstanding Dynamic Binding CapacityAbSolute® High Cap combines the benefits of a new-generation Protein Awith rigid particles providing improved mechanical resistance. Due to a very uniform particle size distribution and a homogeneous pore size distribution, AbSolute® High Cap has outstanding performance at low and high velocities. As an example, DBCs at 10% breakthrough were determined for AbSolute® High Cap and several industry standards with human IgG in PBS at 0.5 mg/mL: AbSolute® High Cap provides much better results than market standards at all linear velocities.
Cleaning and SanitizationAbSolute® High Cap is based on specially modified silica and has multiple-point protein A attachment to the matrix. Its resistance to high pH levels makes it stable through alkaline cleaning and sanitization. Indeed, AbSolute® High Cap remains stable after 400 cycles of use with alkali washing using 100 mM NaOH + 0.5 M NaCl every 10 cycles.
Highest Productivity and EconomicsBased on experimental data obtained at lab scale, an affinity chromatographic step was designed to process 10,000 L of feed at 1.4 g/L of MAb in 16 hours. AbSolute® High Cap is incompressible meaning that a 1-L column is packed with 1 L of media. This feature represents an economical benefit in comparison with soft gels such as agarose-based media, which have compression factors of 1.25 and thus require a higher quantity of media to pack a 1-L column.
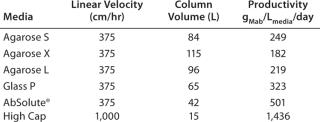
The geometry of all columns has been optimized, thus minimizing the volume of protein A required to perform the step, in order to maximize productivity. In these conditions, performing the step at industrial scale using AbSolute® High Cap allows a volume reduction in the media and an increase in productivity by up to 2.7-fold compared with other industry standards, resulting in a cost savings of up to $1,000,000 for the first load of media!
Table 1: Productivity results for industrial applications
Author Details
Hélène Chochois is product manager biopharma, 81 Boulevard de la Moselle, BP 50, 54340 Pompey, France; +33-3-83-71-47;