
DSM Biologics has developed a highly intensified cell culture process termed XD®, which provides cells with a constant environment for optimal cell growth (Figure 1). The XD® Technology works in a continuous media feeding mode with a filtration unit to retain both the cells and the recombinant protein in the bioreactor. Compared with a standard fed-batch process, the feeding regime in the XD® process can be performed with basal media, and this allows straightforward implementation without the need for extensive feed development. The XD® process is robust and scalable while still maintaining consistent product quality.
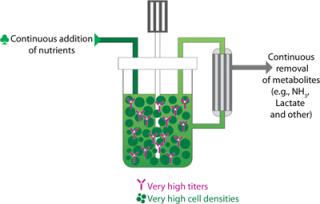
The XD® Technology produces very high cell densities while still retaining high cell viability at the end of the batch, resulting in very high volumetric productivity. At the end of the cell culture (~14 days), the harvest is processed as a single batch. The technology has been used successfully with all the relevant mammalian production cell lines (CHO, hybridoma, myeloma, and PER.C6® cell line) with 5- to 15-fold increases in recombinant protein titers being achieved.
Cell densities higher than 240 million cell/mL have been achieved with CHO cells, resulting in yields up to 11.5 g/L for recombinant proteins (compared with 1.2 g/L achieved using fed-batch mode) and 13.4 g/L for monoclonal antibodies (compared with 1.4 g/L achieved using fed-batch). Monoclonal antibody titers of 27 g/L have been achieved with other cell lines.
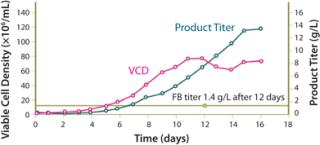

The XD® Technology produces very high cell densities while still retaining high cell viability at the end of the batch, resulting in very high volumetric productivity. At the end of the cell culture (~14 days), the harvest is processed as a single batch. The technology has been used successfully with all the relevant mammalian production cell lines (CHO, hybridoma, myeloma, and PER.C6® cell line) with 5- to 15-fold increases in recombinant protein titers being achieved.
Cell densities higher than 240 million cell/mL have been achieved with CHO cells, resulting in yields up to 11.5 g/L for recombinant proteins (compared with 1.2 g/L achieved using fed-batch mode) and 13.4 g/L for monoclonal antibodies (compared with 1.4 g/L achieved using fed-batch). Monoclonal antibody titers of 27 g/L have been achieved with other cell lines.
Author Details
Rolf Douwenga is vice president of global R&D for DSM Biologics, Zuiderweg 72/2, 9744 AP Groningen, The Netherlands; 31-50-5222-222; rolf.douwenga@dsm.com, www.dsmbiologics.com.
