Traditional influenza vaccine production uses fertilized hen eggs. This method is labor-intensive and requires large facilities with limited scalability. A switch to cellculture–based vaccine production methods and use of disposable products would provide flexibility to substantially reduce the time to vaccine clinical trials and approval, effectively decreasing the time-to-market.
The aim of this work was to establish a process to propagate influenza virus in Vero cell culture using animal-component–free conditions for cell growth and disposable products. The end result would be a fast and simple process enabling scale-up and adaptation to industrial production. The following parameters were evaluated: medium composition, cell detachment, medium supplements, agitation conditions, and infection conditions. After optimization was complete, the robustness and reproducibility of the entire workflow for virus propagation in Vero cells using microcarriers and the WAVE Bioreactor* system were investigated.
Optimization of Cell Attachment and Growth
Studies in T-flasks showed that Vero cells grown in optiPRO** SFM doubled faster than cells grown in VP-SFM. In addition, cultures treated with the recombinant protease TrypLE** Select had a slightly better morphology than those treated with Accutase**.
Vero cell attachment and growth on microcarriers were evaluated in 24-well plates. We observed microscopically that cells were evenly distributed after 48 hours, with the microcarriers approaching confluence. In a separate experiment, when comparing intermittent and continuous rocking conditions in a WAVE Bioreactor system, Vero cell distribution was more even using continuous rocking conditions.
Vero cell growth in a WAVE Bioreactor system was evaluated with media supplements. Pluronic** F-68 protects against shear forces and is often used in WAVE Bioreactor systems. Soy peptone replaces animal serum and is an excellent source of vitamins and carbohydrates. Soybean trypsin inhibitor is used to inhibit tryptic activity. In our experiments, the following supplement concentrations were found to be optimal in a 2-L culture when using 100 mL of 1 mg/mL TryplE for cell detachment: 0.2% Pluronic F-68; 0.3% soy peptone; and 20 mg of soybean trypsin inhibitor.
Optimization of Infection Conditions
Tissue culture infectivity dose50 (TCID50) calculations (1) were used to determine the titer of influenza A/Solomon islands/3/2006 (H1N1) IVR145 WHO (egg-derived) virus, which was adapted through a number of passages in Vero cells.
Studies in T-flasks indicated that, with respect to both cytopathic effects (CPE) and virus titer, the most favorable conditions for infection were a trypsin concentration of 12.5 µg/mL and a virus dilution of 1:1,000. Multiplicity of infection (MOI) was calculated as 0.004 based on the virus stock titer of 106.9, virus dilution of 1:1,000, and cell density of 1.1×106 cells/mL.
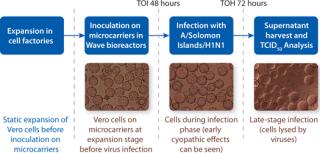
A 2-L batch of influenza virus was prepared in a WAVE Bioreactor system using a disposable Cellbag* bioreactor, Cytodex* 1 microcarriers at 3 g/L, OptiPRO SFM supplemented as described above, and rocking conditions of 12 rpm/5° for 24 hours, then increased to 14 rpm/5° until harvest. The starting vero cell density was 0.4×106 cells/mL, and the infection parameters were MOI, 0.004; time of infection (TOI) 48 h; and temperature 37 °C. Microcarriers were nearly confluent 48 hours after inoculation. Early CPE were seen 24 hours after infection, and cells were detached from the microcarriers and lysed by the virus 72 hours after infection.
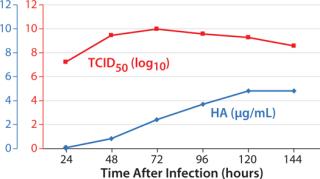
Samples were taken at different time points after infection, from 24 to 144 hours. The concentration of hemagglutinin (HA) was measured using a Biacore* assay (2), and the infectious titer was analyzed using TCID50. Figure 1 shows the results. Infectivity peaked relatively early (72 hours after infection), whereas HA concentration increased slowly for several days.
Robustness and Reproducibility
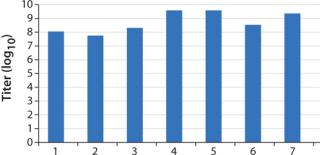
To investigate robustness and reproducibility of the entire workflow, several 2-L batches were prepared in WAVE Bioreactor systems using the conditions described above with a time of harvest (TOH) at 72 hours. All batches were analyzed using a TCID50 assay (Figure 2). The batches had TCID50 values between 107.5 and 109.5.
Conclusions
The aim of this work was to establish a process to propagate influenza virus in Vero cell culture using disposable products and animal-component–free conditions for cell growth. It is important from a regulatory perspective to eliminate animal components in human vaccine production, and it is critical to live virus vaccines because there are limited options for inactivating adventitious virus. We have been able to grow these cell cultures in animal-component–free conditions while providing a fast and simple process that enables scale-up and adaptation to industrial production. We believe that the production of influenza virus in WAVE Bioreactor 20/50 system using a disposable Cellbag is a fast and convenient alternative to stainless steel bioreactors because it is easy to set up and no cleaning is required.
The optimal toH based on infectivity peaked relatively early after infection. For a splitor subunit-based vaccine, infectious activity is not relevant, so it is possible to harvest later and obtain the maximum amount of viral antigen (in this case, HA). In this study, we did not investigate the effects of scale-up.However, all methods and equipment used here are currently used for commercial large-scale vaccine production.
Calculations based on TCID50
values indicate that ~50,000 doses of monovalent live influenza virus vaccine could be produced from a 2-L culture assuming a total process yield of 20% and a dose of 107 infectious virus particles. Thus, a 100-L reactor should hypothetically be able to produce 2,500,000 doses of monovalent live vaccine. A simple workflow of the optimized vaccine production process is shown in Figure 3.
GE, imagination at work and GE monogram are trademarks of General Electric Company. * Biacore, Cellbag, Cytodex and WAVE Bioreactor are trademarks of GE Healthcare companies. ** All third-party trademarks are the property of their respective owners. © 2011 General Electric Company – All rights reserved. First published July 2011.
Author Details
Ann-Christin Magnusson is a senior research engineer, Therese Lundström is a research engineer, and Mats Lundgren is a staff scientist in the R&D department at GE Healthcare Life Sciences Björkgatan 30, SE-751 84 Uppsala, Sweden; ann-christin.magnusson@ge.com; www.gelifesciences.com.
REFERENCES