Research and early process development generally rely heavily on screening and examination of cultures grown in microtiter and shake-flasks. Unfortunately, the growth environment in these formats is extremely different from the bioreactor conditions used in larger-scale production. This application note describes two tools for improving growth conditions at these scales.
Theµ-Flask: Cultures grown in traditional microplates are usually sealed using simple plastic lids or adhesive tape seals. Such systems suffer from well-to-well variability, poor gas transfer, excessive evaporation, and cross-contamination. The µ-Flask (Figure 1) is a multilayer, reusable cover that eliminates well-to-well variability and provides high oxygen-transfer rates for any mictoplate (96-24-, or six-well plates, either deep or shallow).
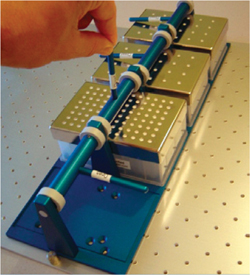
Figure 1:
The RAMbio® System: Cultures grown on orbital-shaking platforms are often oxygen limited, and inadequate oxygenation can lead to inconsistent or suboptimal performance. Orbital-shaking platforms mix cultures to a high degree, but headspace refreshment is often inadequate.
The RAMbio® is a highly efficient mixing incubator and flask aeration system (Figure 2). The ResonantAcoustic® mixing system, combined with the flask-aerating Oxy-Pump® stopper, provides headspace turnover rates and oxygen transfer rates up to sixfold greater than those of typical orbital shakers.

In contrast with other vented flask closures, Oxy-Pump® stoppers actively pump fresh air into flask cultures. When used in the RAMbio®, Oxy-Pump® stoppers deliver up to 2-vvm headspace refreshment rates — a rate equivalent to stirredtank bioreactors. The resulting high oxygen transfer rates allow for fully aerobic growth in shake flasks … and provide for uncompromised levels of biomass production and protein expression. The µ-Flask Provides Uniform Culture Conditions
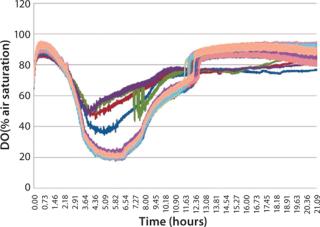
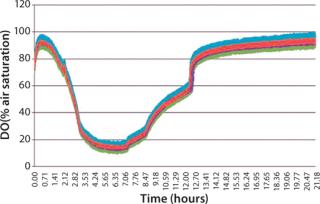
When identical cultures were grown in each well of a microplate using a standard polystyrene lid, the dissolved oxygen level in each well was not uniform over time (Figure 3). This variability is due to differing diffusion lengths and interactions between adjoining wells. The µ-Flask closures eliminate this variability, and each culture exhibits identical dissolved oxygen profiles during growth (Figure 4).
Strain, Media, and Methods
Microplate Cultures:Escherichia coli DH10B grown in 1-mL Terrific Broth + 0.8% glycerol inoculated from a single-source culture; oxygen levels measured using the Sensor Dish Reader® platform (from PreSens via Applikon, Inc.)
Shake-Flask Cultures:E. coli K12 expressing green fluorescent protein (GFP) in H15 medium, 20–40% fill volumes in 0.25-L and 1-L Erlenmeyer flasks; oxygen levels were measured using the Shake Flask Reader® (from PreSens via Applikon, Inc.), and GFP was monitored by a Guava EasyCyte® flow cytometer (Millipore, Inc.).
The RAMbio® Improves Microbial Growth
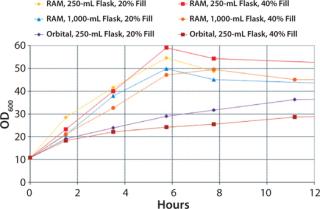
A comparison between cultures grown in the RAMbio® and a standard orbital shaker reveals large differences in OD600 profiles. Cultures grown in the RAMbio® demonstrate accelerated growth up to the final optical densities of 50–60 OD600 within six hours of inoculation (Figure 5). In contrast, cultures grown in baffled shake flasks at 400 rpm demonstrate reduced growth after achieving 18 OD600 and fail to achieve even 40 OD600. The reduced growth is due to oxygen limitation.
The RAMbio® Improves Protein Expression
For many protein expression strategies, successful expression is dependent on aerobic cultivation of the host culture. Following standard procedures for microbial growth in baffled shake flasks (20% fill volume and >300 rpm) cultures often suffer from insufficient aeration. In the data below, the effect of correct culture aeration on recombinant protein expression is visualized by GFP-expressing cultures grown in the RAMbio® (Figure 6).
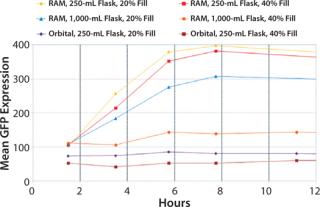
Over-filling of flasks up to 40%
fill volume in a 250-mL flask still demonstrated protein expression superior to that seen in the 250-mL and 1,000-mL baffle flasks with 20% fill volumes grown in an orbital shaker. Only the 1,000-mL RAMbio® flask with a 40% fill volume begins to show reduced expression similar to the orbitally shaken cultures. The RAMbio® Enables Higher Dissolved Oxygen Levels
Maintenance of dissolved oxygen levels is often critical for protein expression in shake flasks for promoting aerobic growth. Figure 7 illustrates the dissolved oxygen profiles for cultures grown using either the RAMbio® or an orbital shaker.
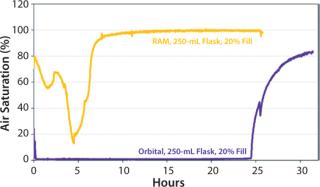
After four hours, the RAMbio® mixing rate was increased, leading to even greater dO2 levels in the RAMbio® cultures. The orbital-flask culture inoculated at 20% fill volume and the same initial density as the RAMbio® culture remains at zero dissolved oxygen throughout the expression experiment (and exhibited poor growth and protein expression). Conclusions
Traditional culturing formats (e.g., microplates or shake flasks) can suffer from variability or unacceptable oxygen limitations. The µ-Flask and RAMbio®+Oxy-Pump® systems promote superior aeration for cultivations compared to older technology. Proper aeration of flask cultures promotes higher growth and can result in increased protein expression levels, and these culture conditions may better reflect strain performance in downstream bioreactor development studies. For more information, contact info@applikonbio.com or call 1-650-578-1396.


The RAMbio® Improves Microbial Growth
A comparison between cultures grown in the RAMbio® and a standard orbital shaker reveals large differences in OD600 profiles. Cultures grown in the RAMbio® demonstrate accelerated growth up to the final optical densities of 50–60 OD600 within six hours of inoculation (Figure 5). In contrast, cultures grown in baffled shake flasks at 400 rpm demonstrate reduced growth after achieving 18 OD600 and fail to achieve even 40 OD600. The reduced growth is due to oxygen limitation.

The RAMbio® Improves Protein Expression
For many protein expression strategies, successful expression is dependent on aerobic cultivation of the host culture. Following standard procedures for microbial growth in baffled shake flasks (20% fill volume and >300 rpm) cultures often suffer from insufficient aeration. In the data below, the effect of correct culture aeration on recombinant protein expression is visualized by GFP-expressing cultures grown in the RAMbio® (Figure 6).

Over-filling of flasks up to 40%
fill volume in a 250-mL flask still demonstrated protein expression superior to that seen in the 250-mL and 1,000-mL baffle flasks with 20% fill volumes grown in an orbital shaker. Only the 1,000-mL RAMbio® flask with a 40% fill volume begins to show reduced expression similar to the orbitally shaken cultures. The RAMbio® Enables Higher Dissolved Oxygen Levels
Maintenance of dissolved oxygen levels is often critical for protein expression in shake flasks for promoting aerobic growth. Figure 7 illustrates the dissolved oxygen profiles for cultures grown using either the RAMbio® or an orbital shaker.

After four hours, the RAMbio® mixing rate was increased, leading to even greater dO2 levels in the RAMbio® cultures. The orbital-flask culture inoculated at 20% fill volume and the same initial density as the RAMbio® culture remains at zero dissolved oxygen throughout the expression experiment (and exhibited poor growth and protein expression). Conclusions
Traditional culturing formats (e.g., microplates or shake flasks) can suffer from variability or unacceptable oxygen limitations. The µ-Flask and RAMbio®+Oxy-Pump® systems promote superior aeration for cultivations compared to older technology. Proper aeration of flask cultures promotes higher growth and can result in increased protein expression levels, and these culture conditions may better reflect strain performance in downstream bioreactor development studies. For more information, contact info@applikonbio.com or call 1-650-578-1396.

The RAMbio® Improves Protein Expression
For many protein expression strategies, successful expression is dependent on aerobic cultivation of the host culture. Following standard procedures for microbial growth in baffled shake flasks (20% fill volume and >300 rpm) cultures often suffer from insufficient aeration. In the data below, the effect of correct culture aeration on recombinant protein expression is visualized by GFP-expressing cultures grown in the RAMbio® (Figure 6).

Over-filling of flasks up to 40%
fill volume in a 250-mL flask still demonstrated protein expression superior to that seen in the 250-mL and 1,000-mL baffle flasks with 20% fill volumes grown in an orbital shaker. Only the 1,000-mL RAMbio® flask with a 40% fill volume begins to show reduced expression similar to the orbitally shaken cultures. The RAMbio® Enables Higher Dissolved Oxygen Levels
Maintenance of dissolved oxygen levels is often critical for protein expression in shake flasks for promoting aerobic growth. Figure 7 illustrates the dissolved oxygen profiles for cultures grown using either the RAMbio® or an orbital shaker.

After four hours, the RAMbio® mixing rate was increased, leading to even greater dO2 levels in the RAMbio® cultures. The orbital-flask culture inoculated at 20% fill volume and the same initial density as the RAMbio® culture remains at zero dissolved oxygen throughout the expression experiment (and exhibited poor growth and protein expression). Conclusions
Traditional culturing formats (e.g., microplates or shake flasks) can suffer from variability or unacceptable oxygen limitations. The µ-Flask and RAMbio®+Oxy-Pump® systems promote superior aeration for cultivations compared to older technology. Proper aeration of flask cultures promotes higher growth and can result in increased protein expression levels, and these culture conditions may better reflect strain performance in downstream bioreactor development studies. For more information, contact info@applikonbio.com or call 1-650-578-1396.
fill volume in a 250-mL flask still demonstrated protein expression superior to that seen in the 250-mL and 1,000-mL baffle flasks with 20% fill volumes grown in an orbital shaker. Only the 1,000-mL RAMbio® flask with a 40% fill volume begins to show reduced expression similar to the orbitally shaken cultures. The RAMbio® Enables Higher Dissolved Oxygen Levels

After four hours, the RAMbio® mixing rate was increased, leading to even greater dO2 levels in the RAMbio® cultures. The orbital-flask culture inoculated at 20% fill volume and the same initial density as the RAMbio® culture remains at zero dissolved oxygen throughout the expression experiment (and exhibited poor growth and protein expression). Conclusions
Traditional culturing formats (e.g., microplates or shake flasks) can suffer from variability or unacceptable oxygen limitations. The µ-Flask and RAMbio®+Oxy-Pump® systems promote superior aeration for cultivations compared to older technology. Proper aeration of flask cultures promotes higher growth and can result in increased protein expression levels, and these culture conditions may better reflect strain performance in downstream bioreactor development studies. For more information, contact info@applikonbio.com or call 1-650-578-1396.
Author Details
Wesley Marner II is an applications specialist at Applikon Biotechnology, Inc., 1180 Chess Drive, Foster City, CA 94404; 1-650-578-1396; wmarner@applikonbio.com; www.applikonbio.com.

