Welcome to the BioProcess International Conference and Exhibition, your one-stop industry resource for driving down costs; improving quality; achieving rapid, robust, and resilient process development and manufacturing. This week you will have an opportunity to explore challenges, uncover solutions, and develop opportunities at the largest and most highly respected event solely dedicated to biopharmaceutical manufacturing.
This year, the conference features more than 50 presentations with new, previously unpublished data as well as many more to choose from, including
- Updates from the US FDA on biosimilars legislation and EMA on a draft guidance for monoclonal biosimilars
- Sessions on managing global manufacturing facilities and partners
- Novel approaches and applications for viral clearance
- In-depth coverage of antibody–drug conjugate development and production.
This annual event also offers an expanded roster of preconference symposia to help you gain new skills and update your knowledge in a variety of areas. Learn from more than 180 case studies and cutting-edge presentations. Consult with more than 150 product and service providers in the exhibit hall. Develop valuable partnerships through rapid-f ire speed networking. And take advantage of continued consultations with fellow attendees through our LinkedIn group online.
With all these opportunities for gaining new knowledge, meeting new people, and having important discussions about the future of the bioprocess industry, we know you will benef it from your attendance at the beautiful Long Beach Convention Center. We appreciate your participation and wish you a wonderful week!
And please stop any IBC staff member and offer your comments about our program. We welcome your feedback to continuously improve this great event.
—Ellen C. King (head of the Bioprocessing Series for IBC Life Sciences) and Barry Walsh (conference director and project leader at IBC Life Sciences)
Special Events
STRATEGY DISCUSSION FORUMS
Tuesday, 10:15–11:45 AM: Integrating New Technologies
1:30–3:15 PM: Global Advances in the Development and Regulation of Biosimilars
Wednesday, 10:30 AM–12:00 PM: The Future of Single Use Technology (Sponsored by ThermoScientific)
10:30 AM– 2:00 PM: Flexibility and Agility as Strategic Purpose, Time and Cost Savers as a Tactical Means to Get There
Thursday,1:45–3:15 PM: Business Models for Biotech–CMO Partnerships (Sponsored by DSM)
Friday, 10:15–11:45 AM: The Industrialization of Single-Use Manufacturing Technology (Sponsored by ATMI, EMD Millipore, Pall Life Sciences, and Sartorius-Stedim Biotech)
SYMPOSIA, MONDAY, 31 OCTOBER 2011, 1:00–5:00 PM
#1: Contamination Control — Lessons Learned, Best Practices and Case Studies
#2: Incorporating Single-Use in Process Development and Scale Up
#3: Understanding Lyophilization and Designing an Optimal Lyophilization Process
#4: Global Strategies and Experience in the Development of Biosimilars
#5: Case Studies of Process and Analytical Transfers in Biomanufacturing
#6: Using DOE Effectively for Process Characterization without Getting Lost in the Statistics
KEYNOTES, PLENARIES, AND FEATURED PRESENTATIONS
Tuesday, 8:00–8:45 AM, Keynote (Formulation track)
Understanding How Solution Conditions Affect Stability by Investigating Site-Specific Changes within Proteins
4:00–5:45 PM,Keynote (Main Conference)
Overview of Approval Pathway under Biologics Price Competition and Innovation Act of 2009
EMA Draft Guideline on Biosimilar Monoclonal Antibodies
Ready or Not Biosimilars Are Coming to US
Wednesday, 1:45–3:30 PM,Plenary Session (Main Conference):
Biotech Approaches to Target Alzheimer’s Disease
Biosimilars: European Experience, mAbs and New Regulations
The Evolving Landscape of Companion Diagnostics in Drug Development
4:15–5:45 PM,Keynotes (Main Conference)
Bioprocess Technology and Innovation: Strategic Imperative for Challenging Times
Integrating Two Biologics Manufacturing Networks
Case Studies: Resolving Formulation Problems
Thursday, 8:00–8:45 AM, Keynotes
Cell Culture track: The International Chinese Hamster Ovary
Genome Project: Where Do We Go from Here?
Special Value-Added track: Vac to the Future: Vaccines
Bioprocessing and Development
Public-Private Partnering for National Security and Public Health Needs
11:30 AM–12:00 PM,Featured Presentation (Recovery track) Fitting the Glass Slipper — Process Design, Plant Capacity and Value Realization
4:45 PM–5:30 PM,Featured Presentation (Cell Culture and Recovery) The Impact of Antibody Drug Conjugates on Process Development and Manufacturing
EXHIBIT HALL HOURS
Tuesday 3:15–7:15 PM, Wednesday 9:45 AM–7:00 PM,
Thursday 9:45 AM–1:45 PM
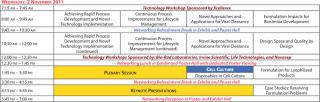
Agenda at a Glance
Please refer to the previous page for details about symposia, keynotes, plenaries, and other special events.
 px;cursor:pointer;” >
px;cursor:pointer;” >