Successful cryopreservation of hematopoietic progenitor cells (HPC) depends on a number of factors: biopreservation media, freezing rates, and storage using sterile, cryogenically durable storage bags. Cell-Freeze® cryogenic storage containers are 510(k) cleared for storage, preservation, and transfer of hematopoietic progenitor stem cells (HPCs). These bags are made from a unique polyolefin film that offers excellent durability while remaining flexible when stored at ultralow temperatures (−196°C). Their proprietary membrane port design offers thinner walls for increased flexibility, and an industry-standard label pocket design lends ease of use and traceability for product labeling. Available interface tubing and connectors are compatible with sterile connection and smart-seal technologies for closed-system applications.
We investigated bioequivalence of a Cell-Freeze® (Charter Medical, Ltd. Winston-Salem, NC) freezing bag to a Cryocyte™ (Baxter Healthcare, Deerfield, IL) freezing bag. The Cell-Freeze® bags were designed to directly replace those now-discontinued Cryocyte™ bags. Data shown here demonstrate that for post-thaw recovery of human HPCs, these bags perform comparably.
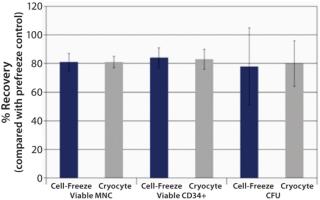
Data SummaryPost-thaw recovery values following storage in liquid nitrogen (LN2)—based on total viable mononuclear cells (MNC), CD34+, and CFU—were observed to be bioequivalent for Cell Freeze® and previously standard Cryocyte™ storage bags (Figure 1). We investigated additional sets of bags for physical integrity. Following six weeks of storage in LN2, frozen bags were removed, exposed to ambient temperature for one minute, and then returned to LN2 for five minutes. Thermal cycling exposure was repeated five times before thawing to simulate a worst-case scenario. All bags passed the physical integrity tests that included both microbial-challenge and dye-immersion tests, indicating no fracturing or breakage (Table 1).
Table 1: Physical integrity testing
For cell preservation assessment, we compared 25 Cell-Freeze® bags from three separate manufactured lots with 25 Cryocyte™ bags from one lot. Bags were filled to the validated maximum freezing fill volume with diluted human HPC product and 10% DMSO at an average 4.64 × 106 cells/mL, frozen in a controlled-rate freezer, and subsequently transferred to LN2 storage for 42-43 days. After thawing, all units were tested for post-thaw viable MNC and CD34+ (as determined by flow cytometry) and CFUs of hematopoietic lineage (by culturing in MethoCult™ medium). For physical integrity, 135 Cell-Freeze® bags (three lots) were investigated. Tryptic soy broth (TSB) containing 10% DMSO was added, and the units were frozen and stored as described above. After cryopreservation, bags were thawed and tested for leakage by pressure and visual inspection before microbial challenge and dye-immersion tests were performed. For microbial challenge, thawed bags were immersed in a bath of B. diminuta followed by 14 days of storage at 30–35 °C. For additional leak testing, bags were immersed overnight in a methylene blue dye bath.
Results and ConclusionHuman HPC post-storage recovery data demonstrate that Cell-Freeze® cryogenic storage bags are bioequivalent to the discontinued Cryocyte™ bags. Average post-thaw recoveries of viable MNC, CD34+, and CFU were comparable. All bags remained intact (no breakage or fracturing) after extensive storage in LN2 and thermal cycling before thawing. So the Cell-Freeze® cryogenic storage containers from Charter Medical, Ltd. offer comparable cell recoveries to Cryocyte™ bags. The unique polyolefin film offers the added benefit of flexibility at cryogenic (−196 °C) temperatures.
Author Details
Dominic Clarke, PhD, is freezing product manager and cell therapy specialist at Charter Medical, 3948-A Westpoint Blvd, Winston-Salem, NC 27103; 336-714-4217; DClarke@Lydall.com