Selecting a CMOis critical to biotech biologic drug development. A key factor for selecting a CMOis experience. How do you measure experience? It can be measured in many ways, metrics include
- Number of GMP processes developed by the CMO
- Number of projects involving your strain
- Number of projects involving your product type
- GMP production success rate
- Number of GMP lots produced.
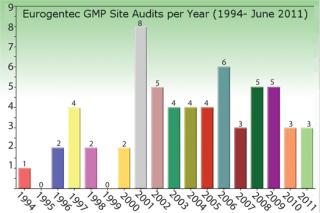
Eurogentec’s Biologics Division is specialized in the manufacturing of biopharmaceuticals from microbial sources such as bacteria, yeast, and biosafety level 2 organisms. Eurogentec manufactures in accordance with GMP since 1994.
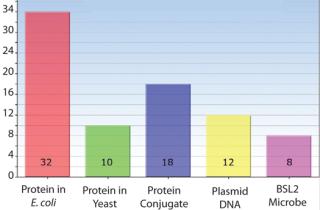
Host Cell Experience — Microbial Experts
- Escherichia coli
- Pichia pastoris
- Hansenula polymorpha
- Saccharomyces cerevisiae
- Numerous BSL-2 organisms.
Chemistry Experience — Conjugation Experts
- Maleimide
- Glutaraldehyde
- Other chemistries available.
Product Family Experience
- Recombinant proteins
- Plasmid DNA
- Protein–PEG conjugations
- Protein–protein conjugations
- Protein–peptide conjugations.
GMP Infrastructure
- Multiproduct facility
- Separate HVAC systems
- Three GMP fermentation suites (80 L, 150 L, 500 L)
- Two GMP purification suites
- One GMP sterile filtration suite.
Process Development Strategies
- FastTrack — quick to clinic
- OptiTrack — robustness built in.
Author Details
Dr. Pascal Bolon is biologics sales and marketing manager at Eurogentec SA, Rue du Bois Saint-Jean 5, 4102 Seraing, Belgium; 32-4-366-6116; biologics@eurogentec.com.