Monitoring for contaminants is a critical step during the production process in the pharmaceutical and medical device industries. Endotoxin, a frequent contaminant, can cause fever, inflammation, headache, nausea, and even death. Found in the cell walls of gram-negative bacteria, endotoxin has routinely been detected by the sensitive and specific Limulus amebocyte lysate (LAL) assay. In the presence of endotoxins, LAL coagulates through an enzyme-mediated cascade, which has then been traditionally quantified based on gelation and turbidity.
The Pyrochrome assay from Associates of Cape Cod, inc. (ACC) contains a synthetic substrate with paranitroaniline chromogen (pNA), which enables spectrophotometric quantitation. Clotting enzyme is (indirectly) activated by endotoxin. The enzyme cleaves the substrate, liberating the pNa, which absorbs light at 405 nm. The absorbance (A405) can be measured by a plate reader and is proportional to the endotoxin concentration in the sample.
Maintaining data integrity is a high priority for companies using instruments/software to capture and store data in environments striving to meet regulatory requirements, including FDA 21 CFR Part 11. Transferring data among multiple software applications for collection, analysis, and presentation can introduce errors and complicate traceability. Additional overhead is also created in supporting, validating, and training personnel on multiple applications.
To validate the VersaMax⢠Absorbance Microplate Reader for use with the Pyrochrome® assay, data acquisition and analysis were performed with SoftMax® Pro 5 GxP Software. Preconfigured method protocols specifically written for the Pyrochrome® assay and other ACC assays are available for SoftMax Pro Software and take advantage of built-in analytical tools for automating passâfail criteria. Additionally, features of SoftMax Pro GxP Software support GLP/GMP guidelines by including user-assigned permission levels to read data, modify settings and formulas, and electronically sign files.
This application note describes a typical set-up for a standard curve in the Pyrochrome® assay. The chromogenic test was conducted using a VersaMax absorbance Microplate Reader. Data were analyzed and graphed using SoftMax Pro GxP Software.
Materials
- VersaMax Microplate Reader (Molecular Devices Catalog No. VERSAMAX)
- SoftMax Pro GxP Software version 5.4 (Molecular Devices Catalog No. SMP54-GXP10UL)
- Pyrochrome® assay chromogenic test reagent (ACC Catalog No. C1500)
- Control standard endotoxin (ACC Catalog No. E010)
- Pyroplate® 96-well microplate (ACC Catalog No. CA961)
Methods
Before dispensing reagents to the microplate, the VersaMax Reader was turned on and SoftMax Pro GxP Software was launched. After selecting the âPyrochrome® Kineticâ method protocol from the drop-down menu in SoftMax Pro GxP Software, a new data file was created on screen.
Test and reagent information can be entered at any time in the designated Notes sections. Entering text and modifying formulas are subject to assigned user permissions. With appropriate user permissions, the plate template assigning samples to the plate layout may also be modified at any time to reflect the proper orientation within the plate. Settings and calculations in SoftMax Pro GxP Software cannot be accessed unless the corresponding permission is assigned to a user.
The incubator temperature was set to 37 °C. The Pyrochrome® assay reagent was reconstituted with 3.2 mL reconstitution buffer, mixing with a gentle swirling motion to avoid excessive foaming and decrease in sensitivity.
- A series of dilutions from the control standard endotoxin solution was prepared in LAL reagent water. The final concentrations used to generate a standard and curve were 50, 5, 0.5, 0.05, and 0.005 EU/mL. 50 µL from each dilution series was transferred in triplicate to the 96-well Pyroplate® microplate, which is certified free of interfering endotoxin and glucan contamination.
- A negative control of 50 µL LAL reagent water was added to the plate in triplicate, and 50 µl of reconstituted Pyrochrome® assay reagent was added to each well as rapidly as possible (optionally with a repeating pipette). Note: Alternatively, 100 µL of each standard/control can be used while maintaining the 1:1 standard:lysate ratio.
The microplate was immediately placed in the microplate drawer, and the measurement process was initiated by clicking on the âreadâ button in the SoftMax Pro GxP Software. The software then automatically performed the following:
- Prior to the read, a proprietary automix motion mixed the contents of each well thoroughly without splashing.
- Absorbance data at 405 nm were acquired every 10â15 seconds for one hour for maximum sensitivity and displayed in real time. The interval and run times can be adjusted as per recommended incubation time for a specific reagent lot number.
- An automatic data reduction was applied to calculate the onset time in each well â that is, the time for each well to reach a specified optical density (OD) threshold. The onset OD specified was set to 0.03 but can be modified.
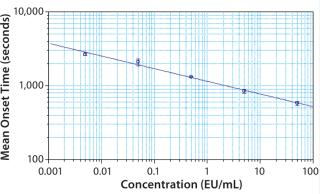
A log-log standard curve was constructed using obtained onset times for concentrations of at least three standards (per USP/FDA). Note: Well assignments must be made through the plate template for the graph to automatically populate. The slope, y-intercept, and correlation coefficient were reported in the graph legend and summary results section.
Results
To demonstrate the suitability of the VersaMax Microplate Reader to perform the Pyrochrome® assay, the resulting standard curve was produced by plotting the log of the mean onset time in seconds against the log of the concentration. The standard curve is noteable in three respects: the high R2 coefficient (0.992), small standard deviations for replicates (y error bars), and the capability of the VersaMax Reader to reach the stated maximum sensitivity of the Pyrochrome® assay (0.005 EU/mL).
The method protocol of SoftMax Pro GxP Software was designed to automatically flag results used to determine the individual assay validity.
- The absolute value of the correlation coefficient â£r⣠should be at
least 0.980. - The positive product control should be within 50â200% of the nominal concentration of the equivalent standard.
- The endotoxin concentration of negative controls (as determined by the extrapolation of the standard curve) should be considerably lower than that of the lowest standard concentration.
Conclusions
The generation of a linear standard curve with low variance between replicates and sensitivity down to the assay’s stated lower limit of detection demonstrates the performance of the VersaMax Microplate Reader and Pyrochrome® assay for detecting endotoxin in test samples. As a result of colorimetric changes, SoftMax Pro GxP Software automatically interpolates the endotoxin concentrations of unknowns from the standard curve based on the onset times. Interpolations can be restricted to the range of onset mean times for the standard curve.
All work carried out in the data file of SoftMax Pro GxP Software is audit trailed. After the results have been reviewed, the file can be locked against further changes regardless of assigned permissions by electronically signing the file. Multiple signature fields are available to indicate various roles, such as reviewer and approver.
The Pyrochrome® assay with the VersaMax Microplate Reader offers a sensitive and specific method for detecting endotoxin contamination in the production process. the all-in- one data acquisition and analysis of SoftMax Pro GxP Software for use with the VersaMax Reader minimizes the risks and costs associated with transferring data among multiple software packages for analysis and reporting.
In addition, SoftMax Pro GxP software helps researchers with GLP/GMP compliance, including the FDA 21 CFR Part 11 requirement for electronic records. The GxP software helps researchers maintain integrity of data by allowing them to control the permissions of authorized users and establish traceability for significant actions.
Author Details
Cathy Olsen, PhD, is an applications scientist, and Craig Tin is a product marketing manager at Molecular Devices, 1311 Orleans Drive, Sunnyvale, CA 94089.