Water is a key raw material used in manufacturing products within the healthcare, pharmaceutical, and biopharmaceutical industries. Microorganisms found in these water systems are mainly stressed, slow-growing strains characterized by long incubation times (five to seven days) before growth can be detected using traditional microbiology methods such as membrane filtration or pour plates. That time required before contamination can be detected in water can cause delays in product release and extend the storage time of products.
Using rapid detection methods, manufacturers can address contamination events sooner, avoid line shutdowns, release product to the market faster, and reduce warehousing costs. This study assesses the ability of the Milliflex Quantum System to detect microbial contaminants in pharmaceutical waters and evaluates its reduction of time-to-result compared with the five to seven days of the compendial method.
Methods and Results
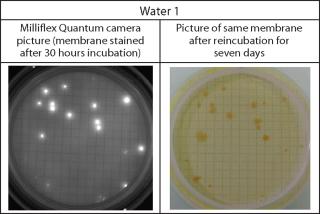
Purified waters are sampled from sampling points from two different processes of a pharmaceutical plant. Purified waters 1 and 2 are immediately diluted and tested with the Milliflex Quantum protocol after 24 and 30 hours of incubation on R2A at 32.5±2.5 °C. These times are tested because previous experiments showed microorganisms normally present in such waters needed 24–36 hours of incubation after the filtration to be accurately detected with the Milliflex Quantum System. Stained membranes are reincubated for a total time of seven days after filtration. Colonies on compendial controls are also visually counted after seven days of incubation.
Membranes are incubated for seven days following a previous experiment with waters from the same sampling plants, proving that seven days were necessary to comfortably visually count colony-forming units (CFUs). Fluorescent counts and CFU counts obtained after reincubation of stained membranes are compared with the Milliflex compendial method. Fluorescent recovery and reincubation recovery parameters are calculated with the following equations:
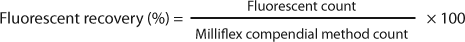

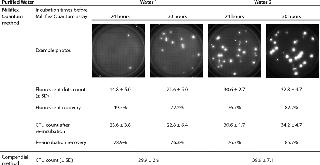
Acceptance criterion for these parameters is set to >70% (Pharmacopeia Forum chapter ). Results are shown in Table 1 and Figure 1.
Table 1:
This experiment clearly shows that a 30-hour incubation is needed to detect > 70% of microorganisms in the purified water 1 with the Milliflex Quantum System. The incubation time before detection with Milliflex Quantum System of purified water 2 can be decreased to 24 hours, with both recoveries equal to 76.7% after 24 days of incubation.
Conclusion
Results of this study show that the Milliflex Quantum System reduces the time to detect water-stressed microorganisms by a factor of five to seven compared with traditional microbiology. Indeed, strains that would normally require up to seven days of incubation are detected after 24–30 hours of incubation.
Because the Milliflex Quantum method is nondestructive, reincubation of stained membranes enables users to identify Delftia acidovorans and Cupriavidus basilensis as the natural contaminants of the water samples using the MicroSEQ® platform. Investigations are then simplified, and implementation of appropriate root cause analysis or corrective/preventive action (CAPA) plans are facilitated.
Dimensions and contrasts of fluorescent dots allow an easy visual count through the Milliflex Quantum Reader, without any background observed on membranes. The fluorescent dots count is similar to the colonies count of the same membrane after reincubation. The ability to quickly detect and enumerate microbial contamination in industrial water systems allows manufacturers to have better process control, higher product yields, and to reduced time to market. Knowledge of contaminants can be used to identify early risks during manufacturing and implement corrective actions. Product release can be accelerated and storage time decreased. This result is an efficient cost saving for manufacturers and an improved quality assurance of their products.
Further Reading
Chapter 2.6.12. Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests. European Pharmacopoeia Sixth Edition, Supplement 6.5: 2009.
Chapter 5.1.6. Alternative Methods for Control of Microbiological Quality. European Pharmacopoeia Sixth Edition, Supplement 6.5: 2009.
General Chapter Microbiological Examination of Non-Sterile Products: Microbial Enumeration Tests. United States Pharmacopeia 32–National Formulary 27: 2009.
General Chapter Validation of Alternative Microbiological Methods. United States Pharmacopeia 32–National Formulary 27: 2009.
Author Details
Hervé Meder, Anne
Baumstummler, and Sophie Rouillon are research scientists, Renaud Chollet is a senior research scientist, and Sébastien Ribault is applied biology R&D manager at Millipore Corporation, 290 Concord Road, Billerica, MA 01821; 1-800-645-5476; www.millipore.com.