Production of biopharmaceuticals has over recent years evolved from simple scale-up of lab-based techniques into a much more engineering-driven task. The entire industry has matured. Safety and efficiency are key drivers in this new era. Large and increasing production volumes of biopharmaceuticals create a need for further development of chromatographic stationary phases. The main focus of resin development has been to increase productivity, which can be achieved by a combination of improved pressure-flow resistance along with increasing the pore diffusion coefficients (dynamic capacity). In combination with sufficient accessible binding sites, higher pore diffusion coefficients result in higher dynamic binding capacities for proteins. Tentacle-based resins such as Fractogel® EMD are known to combine the best of both.
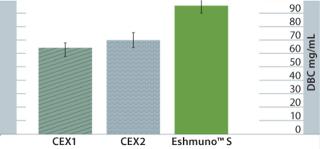
Superior Productivity for MAb CaptureEshmuno™ S strong cation-exchange resin exhibits a superior binding capacity for antibodies than other modern ion exchangers. Figure 1 shows dynamic binding capacity data for direct capture of filtered, conditioned media. The capacity for Eshmuno™ S is ~50% higher than that of other surface-grafted (tentacle) cation exchangers.
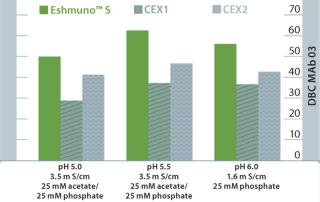
In many commercial processes, the second step in the process is cation exchange to remove HCP and leached protein A. Eshmuno™ S provides superior binding capacity for this crucial application. Figure 2 illustrates increased binding capacity at a variety of feed conditions. In all tested conditions, Eshumno™ S out-performs other tentacle-type resins.
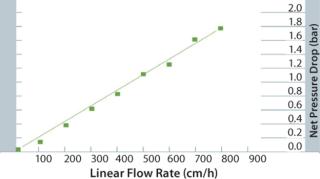
Productivity is a combination of capacity and flow rate. With the exceptional rigidity of the Eshmuno™ base bead, high flow rates can be achieved at the low back pressures needed in large-scale production applications. Figure 3 shows pressure and flow data for a 20-cm ID column of Eshmuno™ S. With the high capacity mentioned above, this results in productivity values of >40 mg/mL per hour, a significant improvement over previous resins.
Eshumno™ S strong cation exchanger is a process resin designed for the most demanding large-scale applications. It has specific utility for the purification of monoclonal antibodies from cell culture — as either a direct capture step or a second, post–protein A polishing operation. For the first time, engineers need not compromise between capacity, selectivity, and flow rate. The result is a resin that can increase process productivity up to fivefold over more conventional approaches. Use of Eshmuno™ S for capture of antibody from cell culture supernatant can reduce purification costs by ≤30% over protein A–based processes.
As with all EMD resins, Eshmuno™ S comes with a complete portfolio of support documents — for packing, cleaning, regulatory filings, and with the individualized support of EMD’s Global Applied Technology staff with centers on three continents and renowned training programs.