Single-use components are finding more use in the biopharmaceutical industry. One application increasing in use is transportation of sterile liquids. There are many challenges to consider when shipping a flexible container with sterile liquids. Typical Bio-Containers used in shipping applications are constructed of multiple layers. The fluid-contact layer is typically constructed of low, linear low, or ultralow density polyethylene (LDPE, LLDPE, or ULDPE). A variety of gasbarrier layers can be used in construction of the multilayer film, and one typical is ethylene vinyl alcohol (EVOH). The outer layer provides strength and can be constructed of polyethylenes, nylons, or polyesters.
Although the film construction is complex and engineered specifically to endure the harsh conditions it may encounter, Bio-Containers manufactured using plastic have property challenges, such as low penetration resistance, when compared with stainless-steel vessels. These containers must be protected during movement inside a manufacturing plant as well as during plant-to-plant transportation. Protective outer containers may range from a portable rack for either hanging or supporting bags in the lay-flat position (for internal plant movements) to fully enclosed totes to protect Bio-Containers from warehouse, truck, and airplane environments.
BackgroundTo ensure that a Bio-Container can withstand the rigors of transportation, an evaluation must be performed. Transportation of a container would typically be subject to blows and impacts in the plant, vibration and collisions on a truck, and vibration and jolts on an airplane. Ideally, one would evaluate such things before designing a bag and support device to find weaknesses in the product(s) before releasing to the market. In the case of a Bio-Container, this is difficult. Charter Medical has chosen to build its bags to fit widely used, commercially available totes to protect them in shipment, which precludes any redesign.
Another factor is the fit of a bag inside a containment device. An undersized bag will not be fully supported at the corners, which will induce additional hydrostatic stresses at rest and hydrodynamic stresses during impacts and vibration. An oversized bag will be fully supported but could have folds or wrinkles that act as stress concentrators. Both situations are difficult to predict and quantify. Forces acting on the bag/tote combination are difficult to calculate, and they vary widely. Furthermore, critical material properties (e.g., fatigue strength and surface factor of a film) used to determine the resistance of a design to fatigue (commonly known as “flex-cracking”) are not readily available for the polymers used — because of the composite nature of films built of different polymers of varying thicknesses, all with diverse mechanical properties. These challenges force product development teams to fully vet a Bio-Container to test it empirically.
Table 1. Transportation test results of four 200-L Clear-Pak™ Bio-Container bags
Testing Bio-Containers for transportation efficacy can be performed in different ways, such as according to a method authored by a standards body (e.g., ASTM or ISTA) or development of an internal company method. Charter’s team chose to follow an ASTM method to take advantage of decades of shipping testing expertise of that standards-writing body. The method chosen was ASTM d4169, Standard Practice for Performance Testing of Shipping Containers and Systems, Distribution Cycle 2 (Schedules A and E), Assurance Level II. Of three possible assurance levels, the midrange was selected as typical for the application.
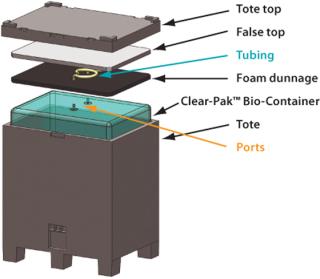
Distribution Cycle 2 designates a specially defined distribution system. The handling and vehicle vibration tests were selected here because the special application would most likely undergo these loads during use at a typical biopharmaceutical plant conscious of the value of product inside the Bio-Container. The handling schedule consists of a number of drops to simulate forklift abuse and other typical handling. The vehicle-testing schedule simulates truck and air shipping load events. The team tested a 200-L three-dimensional Clear-Pak™ Bio-Container in a plastic collapsible commercially available shipping tote. This specific design and similarly sized vessels are used in the industry for plant-to-plant transport of Bio-Containers filled with fluid. The container was tested with ambient-temperature water to simulate aqueousbased solutions.
The acceptance criteria were divided into two categories: first, an integral bag with no leaks; second, no aesthetic defects that could lead to future failure. This assumes that a bag may undergo this amount of stressing in the test regimen and still be required for more movement in the plant, so a defect that appears to be on the threshold of failure could yet propagate into a functional failure at a later processing step.
Once those details were decided, the Bio-Containers were built, gamma-irradiated at standard levels of 25-40 kGy, and shipped in their standard configuration: double-pouched inside of corrugated boxes. Two sets of tests were performed: one of bags filled to capacity and packed with commercially available soft, closed-cell polyethylene foam; one of half-full bags using bubble wrap compressed as dunnage. Both dunnage types were compressed ∼15%. The full bags also used a stiff plastic plate as a false top to reinforce the thin flexible tote top above the dunnage to help prevent possible problems of wave-induced flex-cracking of the film.
After filling and securing of the containers, testing was performed. First came the handling schedule, which consisted of a number of drops. Half-full bags were subjected to a drop height of 9 in., whereas full products were dropped from 6 in. Those drop heights were determined by the weight of the entire container. Next, the units were subjected to the vehicle vibration test schedule: one hour on a vibration table simulating truck transport and two hours simulating air transport. Lastly, a repeat of the handling schedule was performed.
ResultsAfter each test regimen, the tote lid was lifted, dunnage was removed, and the Bio-Container was evaluated according to acceptance criteria. None of the two categories of failure were seen at the tops of the bags or at the inspection ports on the sides of the plastic tote. The bags were continuously evaluated during drainage to look for defects on their sides or bottoms of the bags — and none were found.
SummaryHalf-full and completely full 200-l three-dimensional Clear-Pak™ bags endured transportation testing according to ASTM D4169-05 and passed with no leaks and with no aesthetic defects. Charter Medical’s new product development team recommends that the product and specific shipping configuration detailed in this report be used by end-users for both shipping applications and for in-plant handling.