Developed in 1996, the WAVE Bioreactor™ and Cellbag™ disposable bioreactor systems have revolutionized cell culture for commercial and R&D applications in the production of cell-derived products using animal, insect, plant, microbial, and virus cultures. Oxygen transfer and mixing are accomplished by the novel principle of wave-induced agitation. Wave action generates free surfaces for bubble-free oxygen transfer from the headspace of each Cellbag bioreactor, and it also mixes the fluid suspending cells or particles. This design eliminates the need for mechanical mixing or gas sparging, both of which introduce shear damage to cultures. Air is continuously purged through the bioreactor headspace to supply oxygen and remove metabolic gases. This system can be adapted to control pH and dissolved oxygen or operation of perfusion cultures.
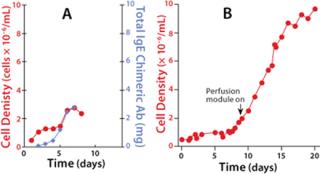
Optimizing Immunoglobulin Expression in a WAVE BioreactorJoseph Mollick, MD, PhD, of the Stanford School of Medicine’s Division of Oncology studies tumor-specific chimeric human IgE expression in CHO-K1 cells. Dr. Mollick’s experience with WAVE technology illustrates how minimal process changes can significantly alter expression profiles. The antibody production runs are inoculated at a seed density of 7×105 cells/mL. The inoculating vessels are Erlenmeyer flasks that have reached a density of 3×106 cells/mL. Inoculum is added directly to the bioreactor and diluted with fresh medium. No medium exchange is necessary.
In the initial runs, the CHO-K1 cells were grown in a serum-free medium as a batch culture with a working volume of 5 L. The cells grew to a maximum of 2.5×106 cells/mL before their viability began to decrease, which was probably due to a buildup of waste products, decreased pH, and nutrient depletion. A perfusion controller (composed of a load cell onto which the reactor is placed) was incorporated, along with pumps that allow for automated fill/harvest operations. In batch mode, maximum cell density was limited due to exhaustion of cell culture medium and was addressed by using continuous media exchange of the cell bag through perfusion. The data in Figure 1b represent a batch culture grown until cell density reached 2×106 cells/mL. At that time, perfusion was initiated, and two-fifths of the total volume was exchanged daily. This approach extended the life of the culture beyond 20 days. Total cell density increased nearly fivefold over the batch culture.Figure 2
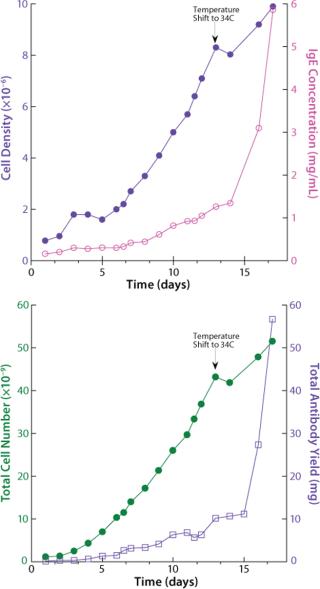
The next run addressed antibody expression levels in the WAVE Bioreactor perfusion system. The original perfusion parameters yielded slightly more than 1 mg/mL. A modification was introduced to the temperature set point. On day 13, the temperature was lowered from 37 °C to 34 °C, which led to a significant sixfold increase in protein levels, reaching 6 mg/mL.
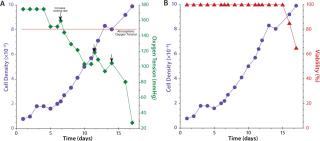
The arrows in Figure 3A illustrate the inversely proportional relationship between cell density and dissolved oxygen (DO) levels. This perfusion culture delivered ambient air (20% oxygen) to the headspace of a Cellbag bioreactor, which was sufficient to support cell densities of 10×106 cells/mL. However, viability of the culture began to decrease on day 15 in a manner possibly dependent on do levels (Figure 3b). One approach to increase oxygen delivery is to increase the rocking rate. However, to prevent significant density-dependent drops in oxygen, use of an O2MIX controller unit providing an environment of up to 50% oxygen is advised. Arrows in Figure 3(left) indicate where the rocking rate was increased to a maximum of 28/min. Notice an immediate increase in oxygen tension at each of those three points.
How has WAVE technology increased the productivity of cell cultures over the previous method? Densities and expression levels are significantly greater in the WAVE Bioreactor than in Erlenmeyer flasks. In shake flasks, cell density is limited to 3×106 cells/mL. In a volumetric sense, WAVE Bioreactors allow cultures at 50% of the total volume of the bag, whereas the shake flasks previously used allowed only 50-mL volumes of culture in each 300 mL flask (16% total volume). Initial expression levels were as low as 0.3 mg/mL before optimization and 6 mg/mL after introduction of WAVE technology.